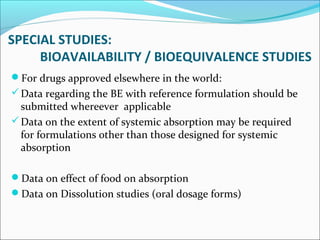
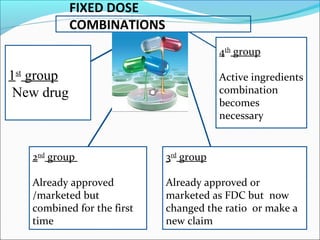
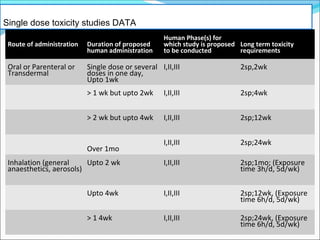
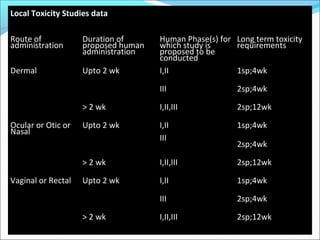
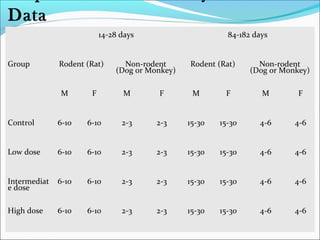
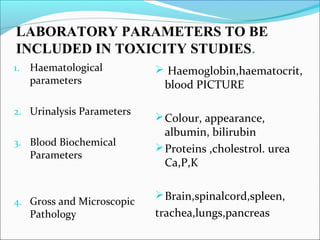
This document provides guidelines for conducting clinical trials and importing or manufacturing new drugs for sale according to Schedule Y of the Drugs and Cosmetics Act of 1940 in India. It outlines the application process and required data to be submitted, including chemical/pharmaceutical information, pre-clinical toxicology and pharmacology studies in animals, protocols for human clinical trials through various phases, and post-marketing surveillance requirements. Ethics committee approval and informed consent of participants are necessary. Manufacturers must establish the drug's quality, safety, and efficacy primarily through clinical trials conducted in India.