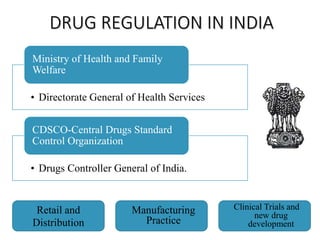
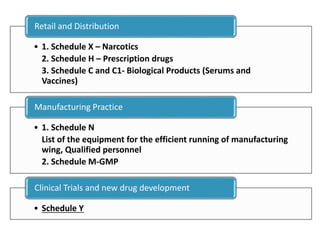
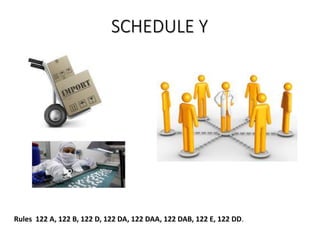
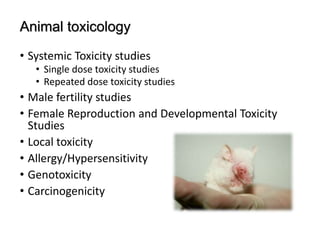
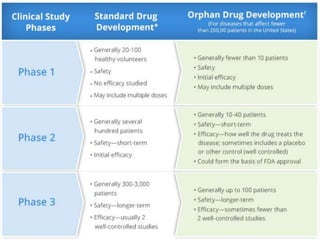
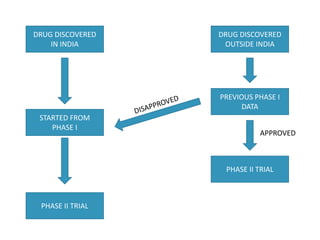
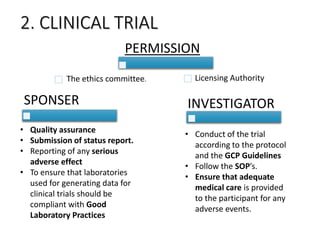
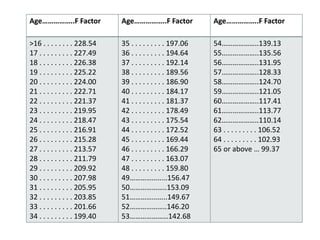
The document provides an overview of drug regulation in India, focusing on the Drugs and Cosmetics Act of 1940. It details various schedules related to drug categories, manufacturing practices, and clinical trial protocols, including the requirements for safety and efficacy studies for new drugs. The document emphasizes the ethical considerations and necessary approvals for conducting clinical trials, as well as the responsibilities of ethics committees in safeguarding trial subjects.