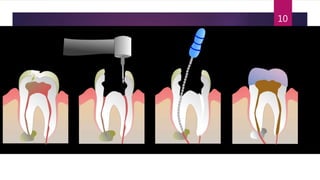
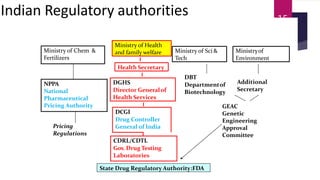
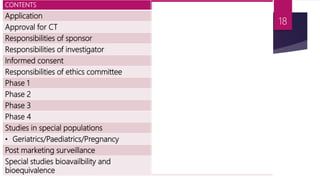
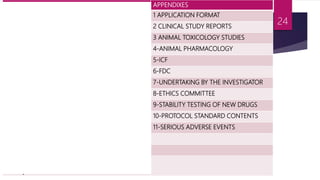
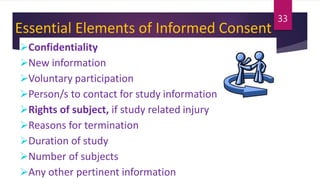
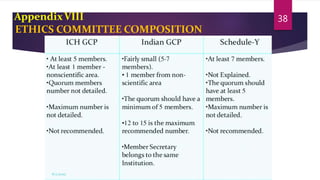
Schedule Y outlines the regulatory requirements for conducting clinical trials in India. It provides guidelines on applying for trial approval, sponsor and investigator responsibilities, informed consent, trial phases and types of studies. The document discusses amendments made to Schedule Y over time to align it more closely with ICH-GCP guidelines. It also describes the 11 appendices that provide details on topics like pre-clinical data submission, animal studies, ethics committee composition, serious adverse event reporting, and stability testing. The goal of Schedule Y is to improve clinical trial quality and ensure data standards are globally accepted.