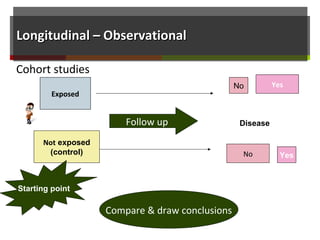
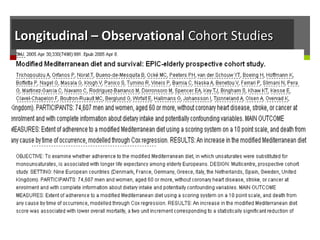
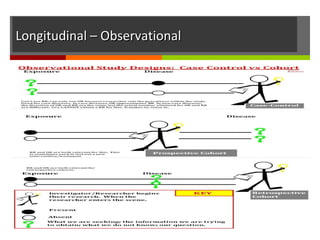
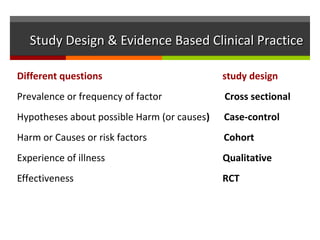
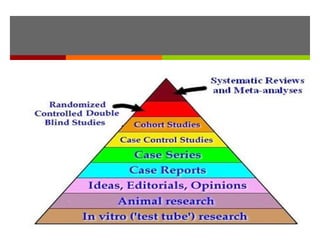
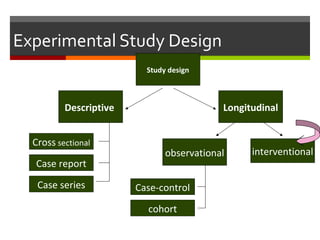
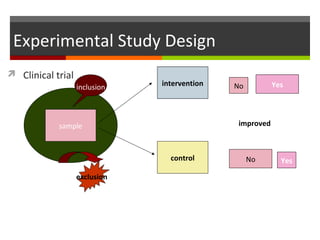
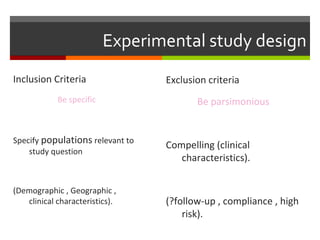
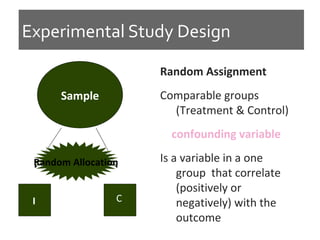
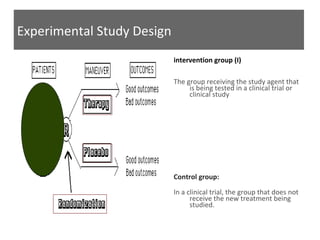
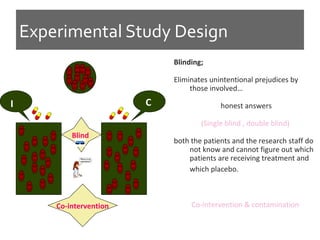
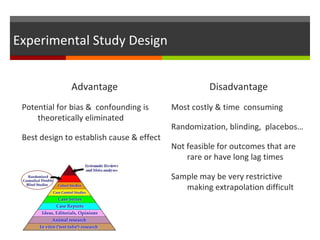
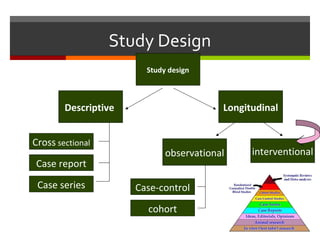
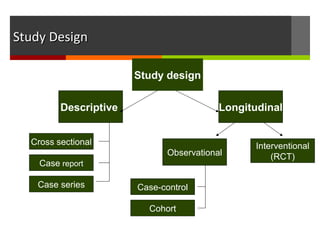
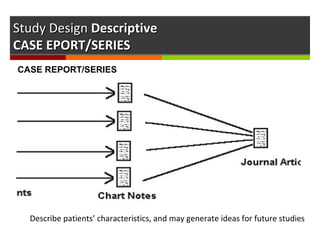
This document discusses different types of epidemiological study designs, including descriptive and experimental designs. Descriptive designs include cross-sectional studies, case reports, case series, and qualitative studies. Cross-sectional studies examine the relationship between diseases and other factors in a population at a single point in time. Case reports and case series describe characteristics of individual patients or small groups. Experimental designs include randomized controlled trials (RCTs) which test the effectiveness of interventions by randomly assigning participants to intervention or control groups. Observational studies like case-control and cohort studies examine the relationship between exposures and outcomes without intervention. The document outlines advantages and disadvantages of each design.







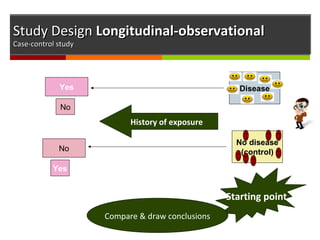

![Study DesignStudy Design Longitudinal - observationalLongitudinal - observational
Case-control studyCase-control study
Disadvantages of case – control studies
Uncertain if exposure preceded disease
Potential for recall bias (sick people may be more [or less]
likely to recall exposure)
Selection bias (recruitment influenced by exposure)](https://image.slidesharecdn.com/rssstudydesign-130615134155-phpapp01/85/RSS-study-design-15-320.jpg)