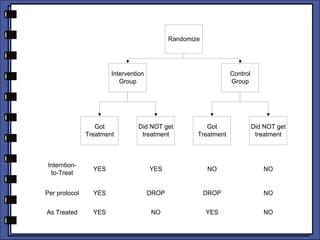
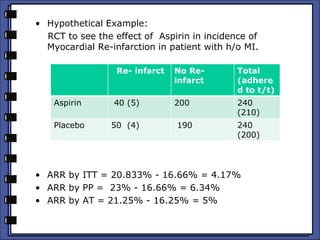
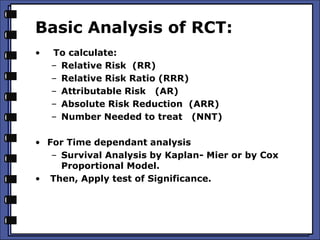
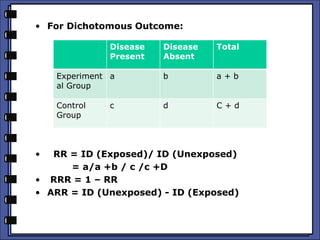
The document discusses the analysis of randomized control trials (RCTs) focusing on comparison methods such as intention-to-treat (ITT), per-protocol (PP), and as-treated (AT) analysis, addressing their advantages and limitations. It highlights the importance of maintaining randomization for statistical validity while noting issues such as protocol violations and loss to follow-up. Various statistical metrics are defined and exemplified through hypothetical RCT outcomes, alongside references for further reading.

![• Attributable Risk
= (OR – 1) PE / 1+ [ (OR-1) PE] x 100
• Where OR = Odds Ratio = ad / bc
• Number Needed to treat (NNT) = 1/ARR
• RR = 0. 4 /0.5 = 0.8
• RRR = 0.2
• ARR = 0.2 – 0.25 = - 0.05
• NNT = 1/ARR = 20
TB No TB Total
Cont.
Isoniazid
40 160 200
Isoniazid 6
month
50 150 200](https://image.slidesharecdn.com/analyzingtherandomisedcontroltrialrct-151214111248/85/Analyzing-the-randomised-control-trial-rct-4-320.jpg)