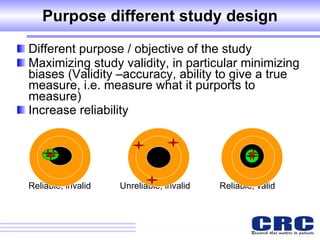
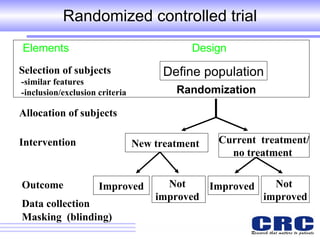
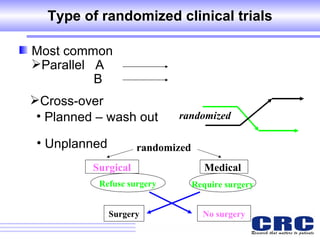
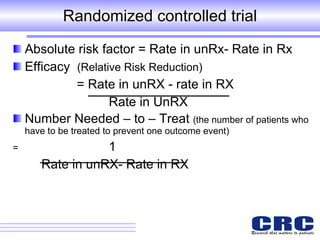
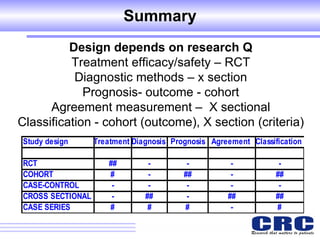
1. Different study designs are used for different research purposes, with randomized controlled trials being strongest for evaluating new therapies but also most difficult to conduct.
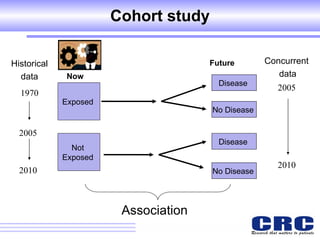
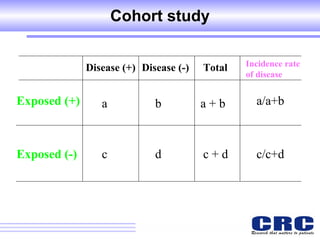
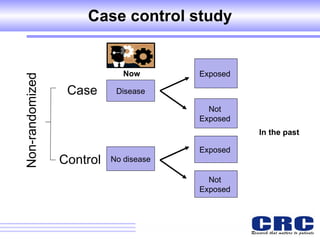
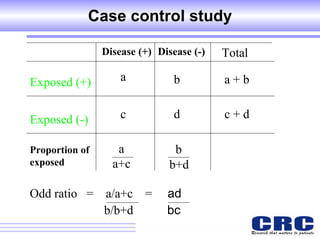
2. Observational studies like cohort and case-control studies can be used when randomization is not possible or ethical, and allow studying rare diseases or past exposures, but are more prone to biases.
3. Qualitative research uses methods like interviews and observations to understand complex social phenomena and issues that are difficult to study quantitatively. The appropriate study design depends on the research question and objectives.