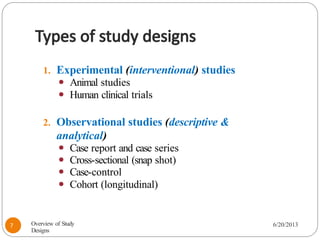
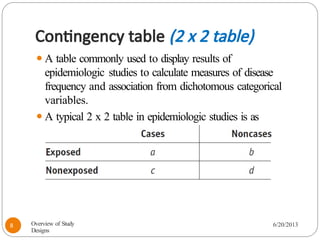
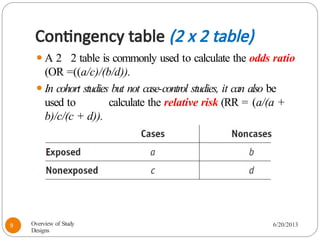
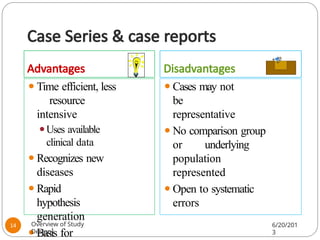
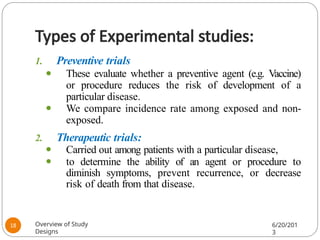
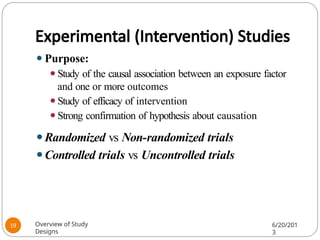
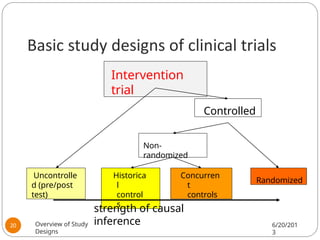
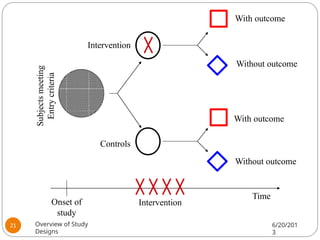
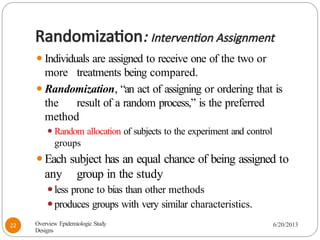
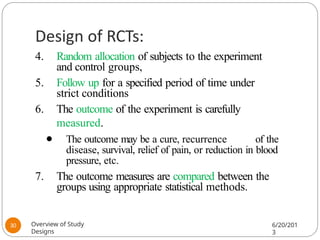
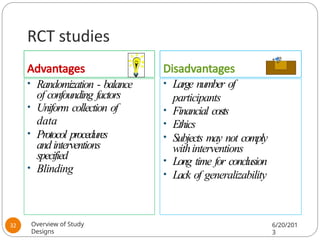
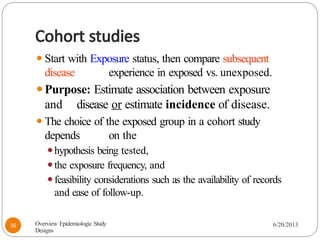
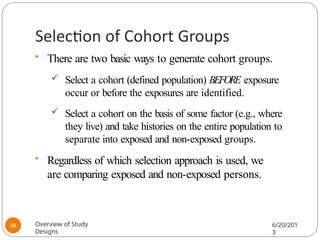
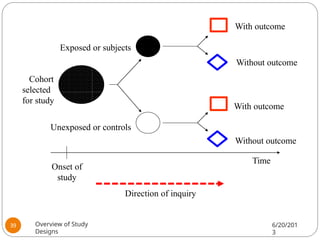
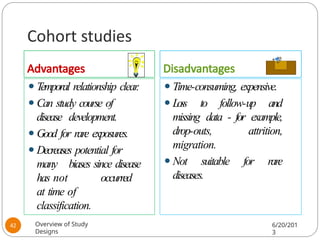
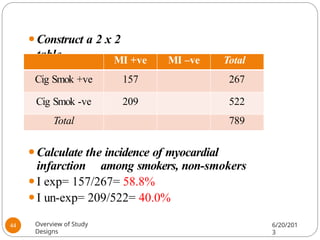
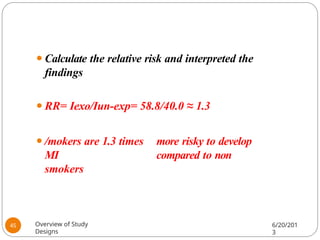
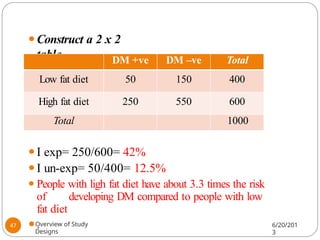
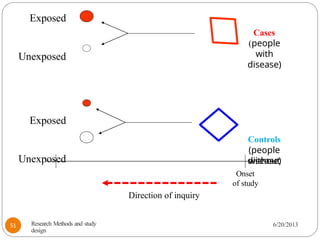
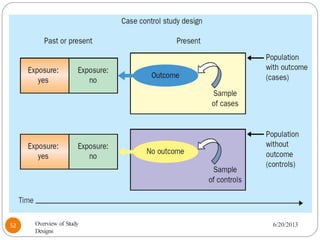
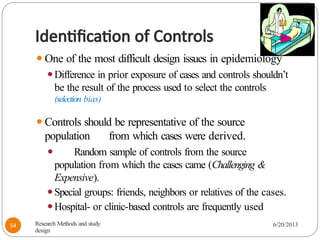
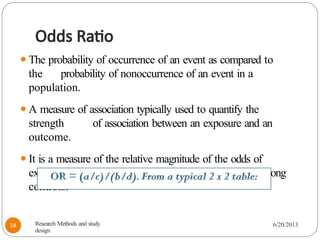
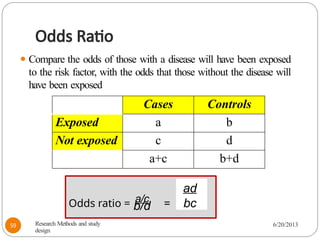
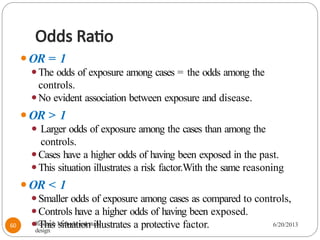
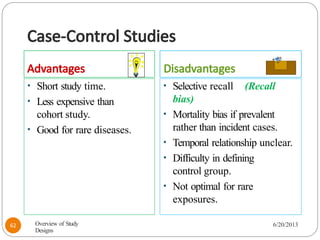
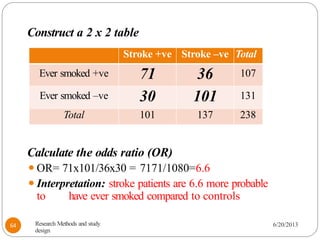
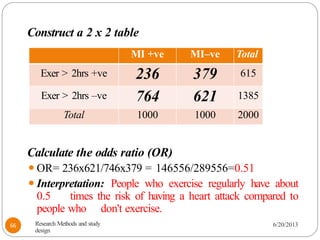
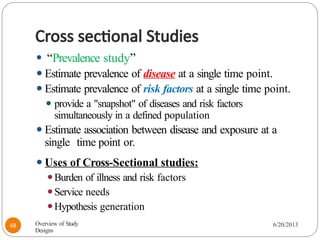
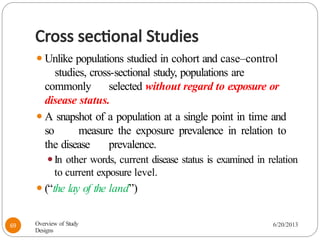
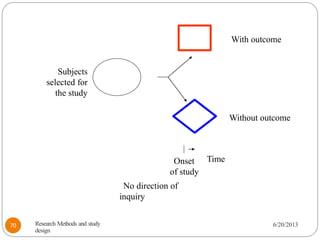
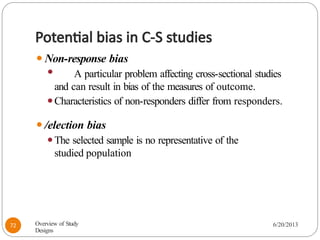
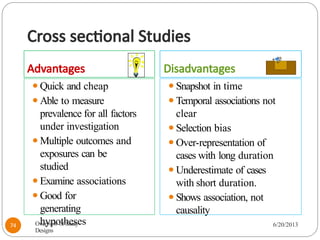
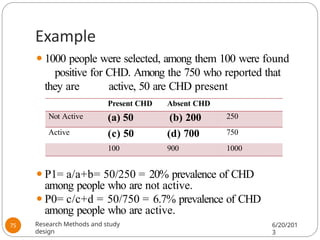
The document provides an overview of various study designs in epidemiology, detailing different types of research methods including experimental and observational studies. It discusses the goals of research, strengths and limitations of different designs, and emphasizes the importance of understanding the temporal relationships between exposure and outcomes. Key concepts such as case reports, cohort studies, randomized controlled trials, and methods of data collection and analysis are also covered.