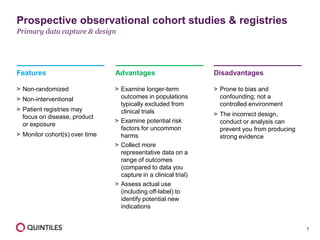
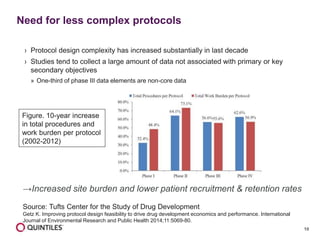
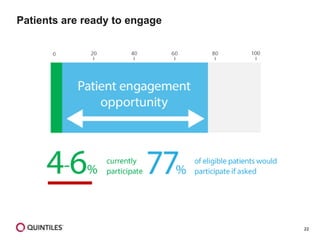
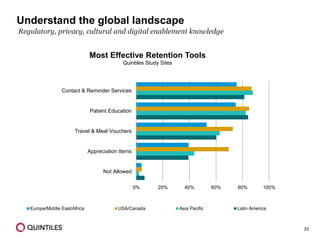
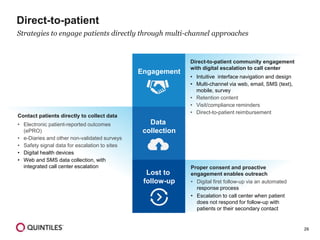
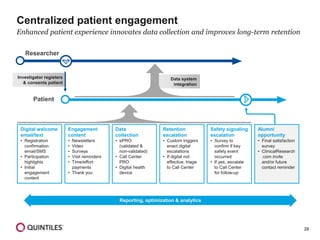
The document outlines the design and operational considerations for conducting long-term observational studies, emphasizing the importance of site and patient engagement for successful retention and compliance. It discusses challenges faced in retaining participants and provides strategies for improving engagement through simpler protocols, effective communication, and targeted outreach. Additionally, it highlights the benefits and limitations of observational cohorts compared to traditional clinical trials, advocating for a streamlined approach to data collection that prioritizes meaningful outcomes.