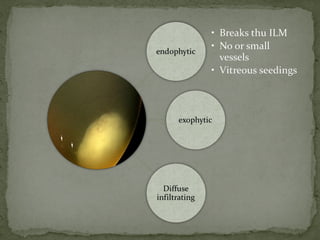
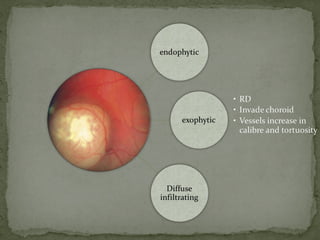
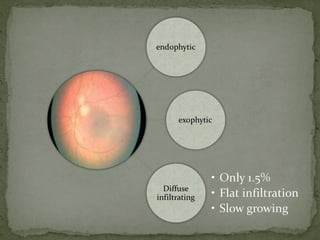
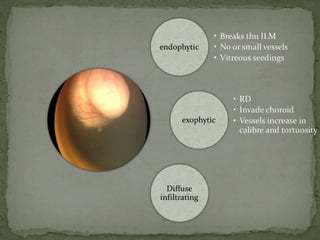
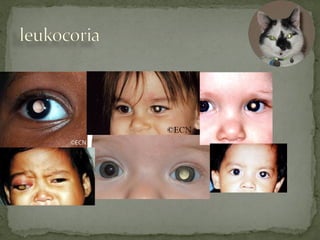
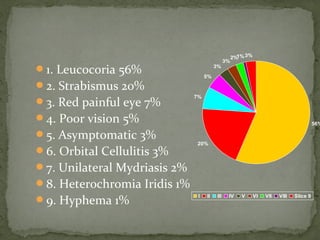
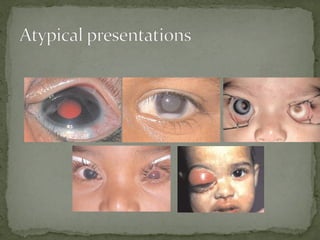
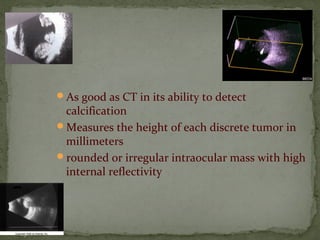
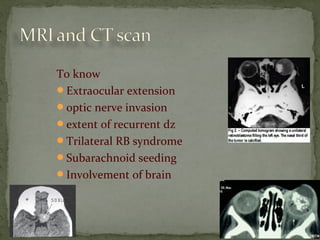
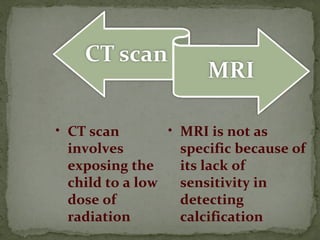
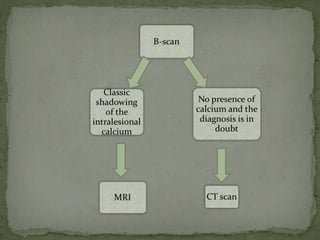
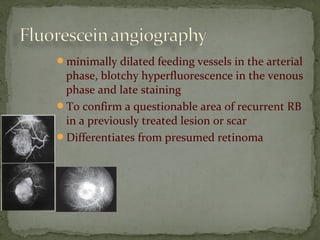
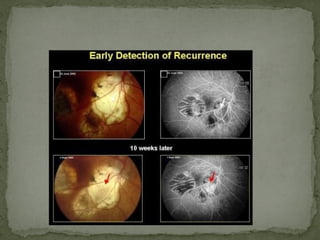
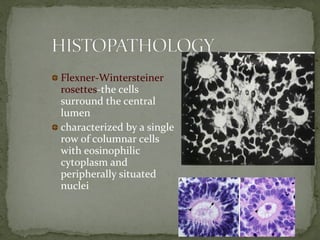
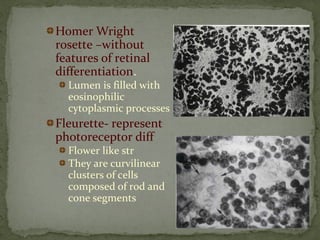
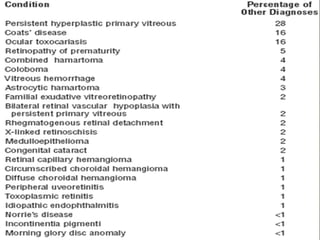
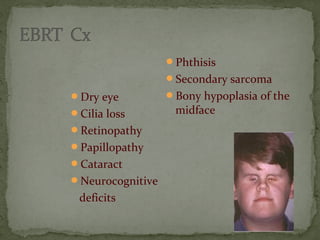
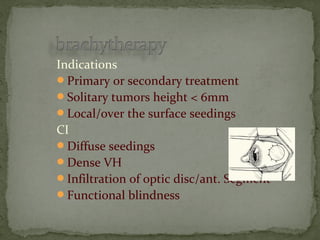
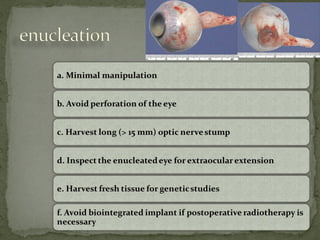
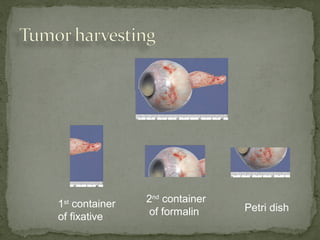
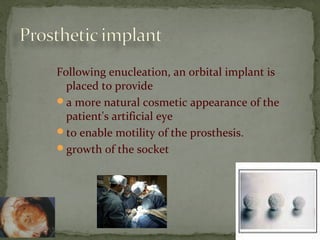
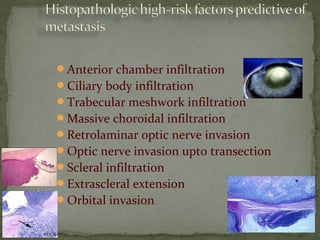
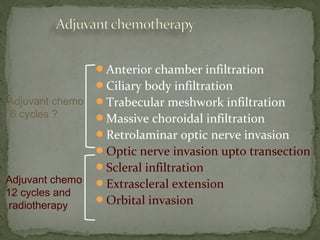
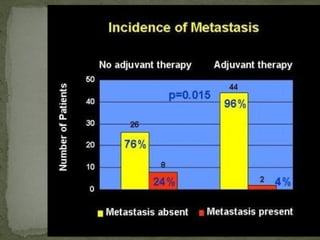
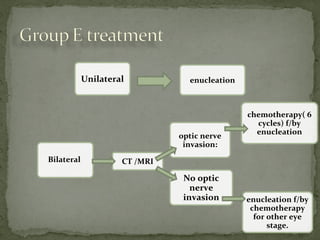
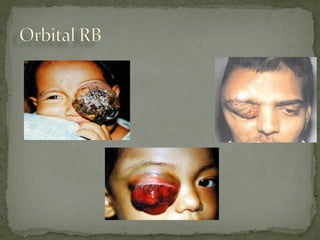
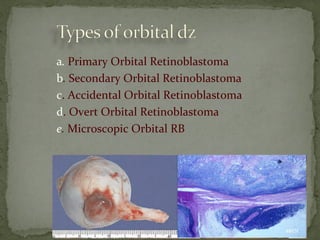
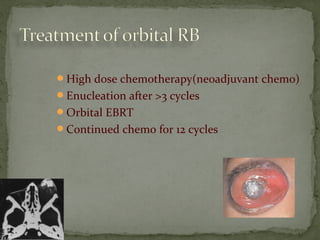
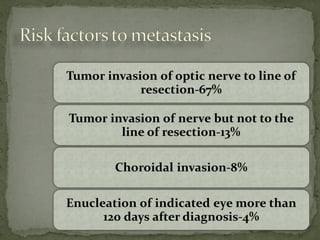
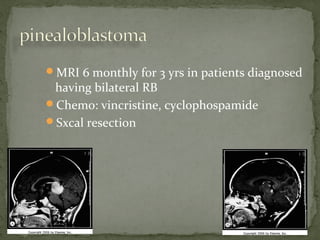
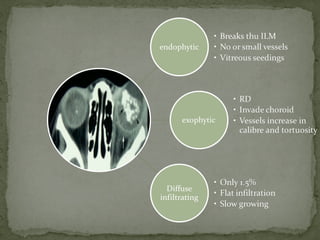
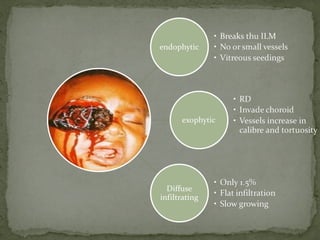
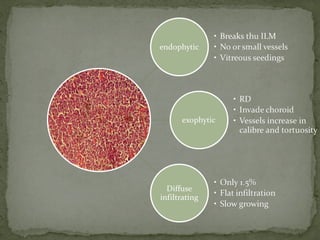
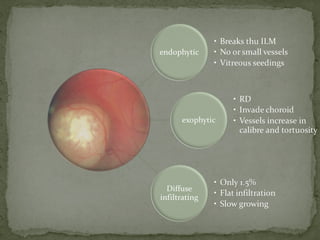
This document provides an overview of retinoblastoma, including:
1. A brief history of retinoblastoma classification and descriptions.
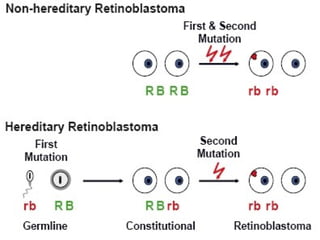
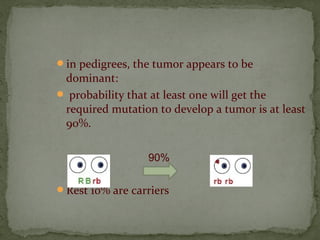
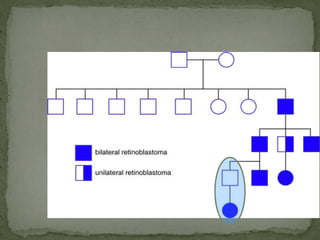
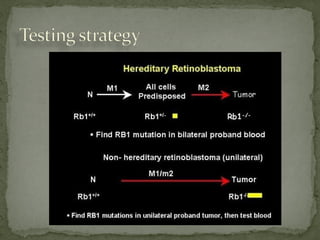
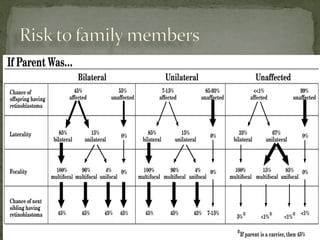
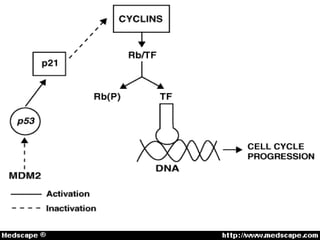
2. Details on the genetics and pathogenesis of retinoblastoma, including the two-hit hypothesis.
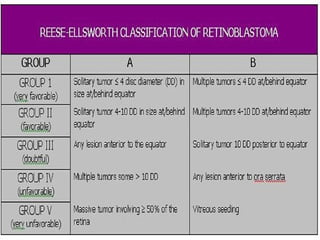
3. Presenting features, diagnostic testing, classification systems, and treatment options for retinoblastoma such as chemotherapy, radiation therapy, cryotherapy, and enucleation.
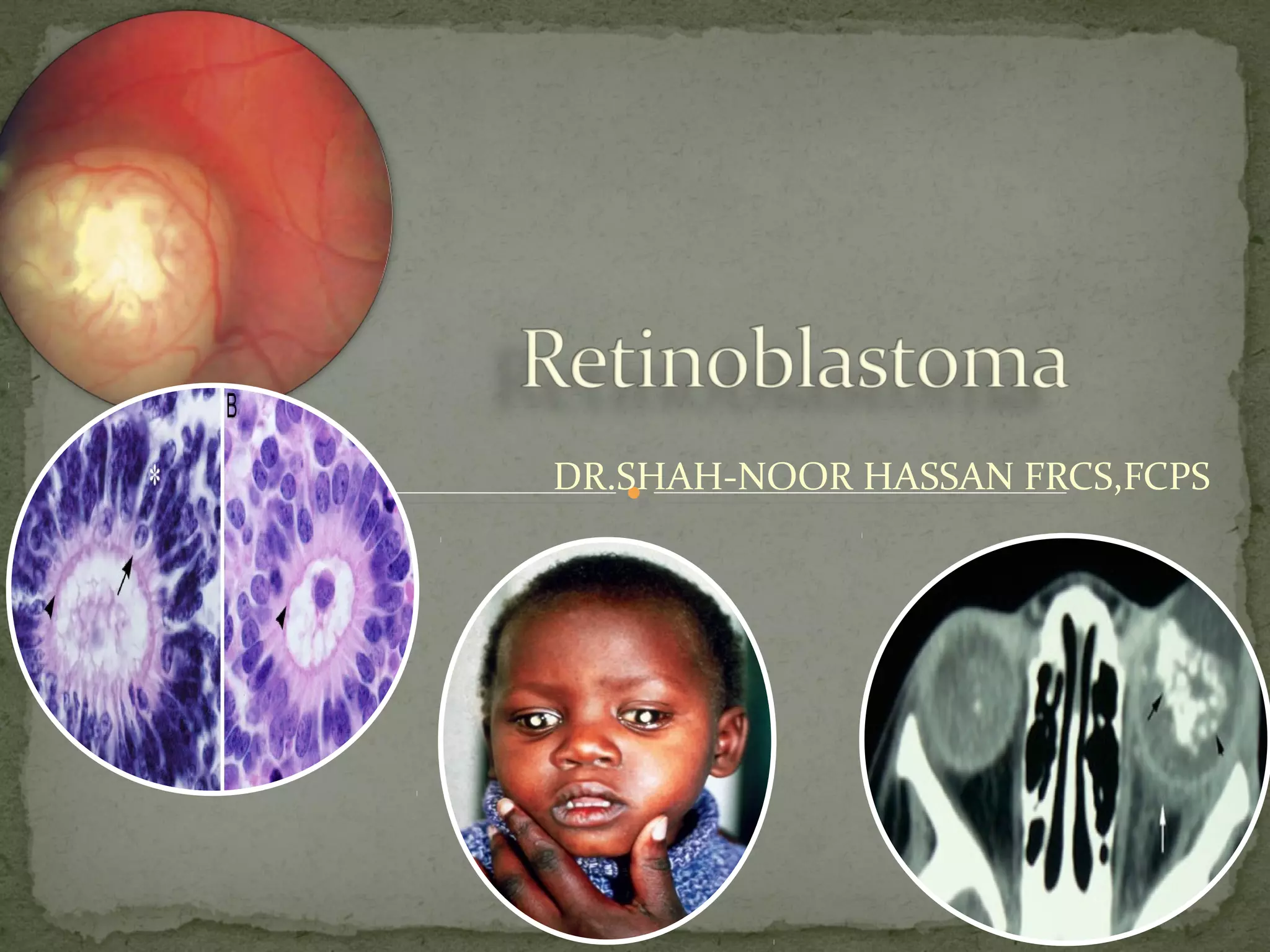


![Retinoblastoma is a rare childhood cancer.
third most common cancer overall affecting
children
3% of all cancers in children younger than 15
years of age
National Cancer Institute [NCI], 2007
most common intraocular malignant neoplasm
in children (Castillo and Kaufman, 2003).](https://image.slidesharecdn.com/retinoblastomapre-180318103316/85/Retinoblastoma-Rb-4-320.jpg)