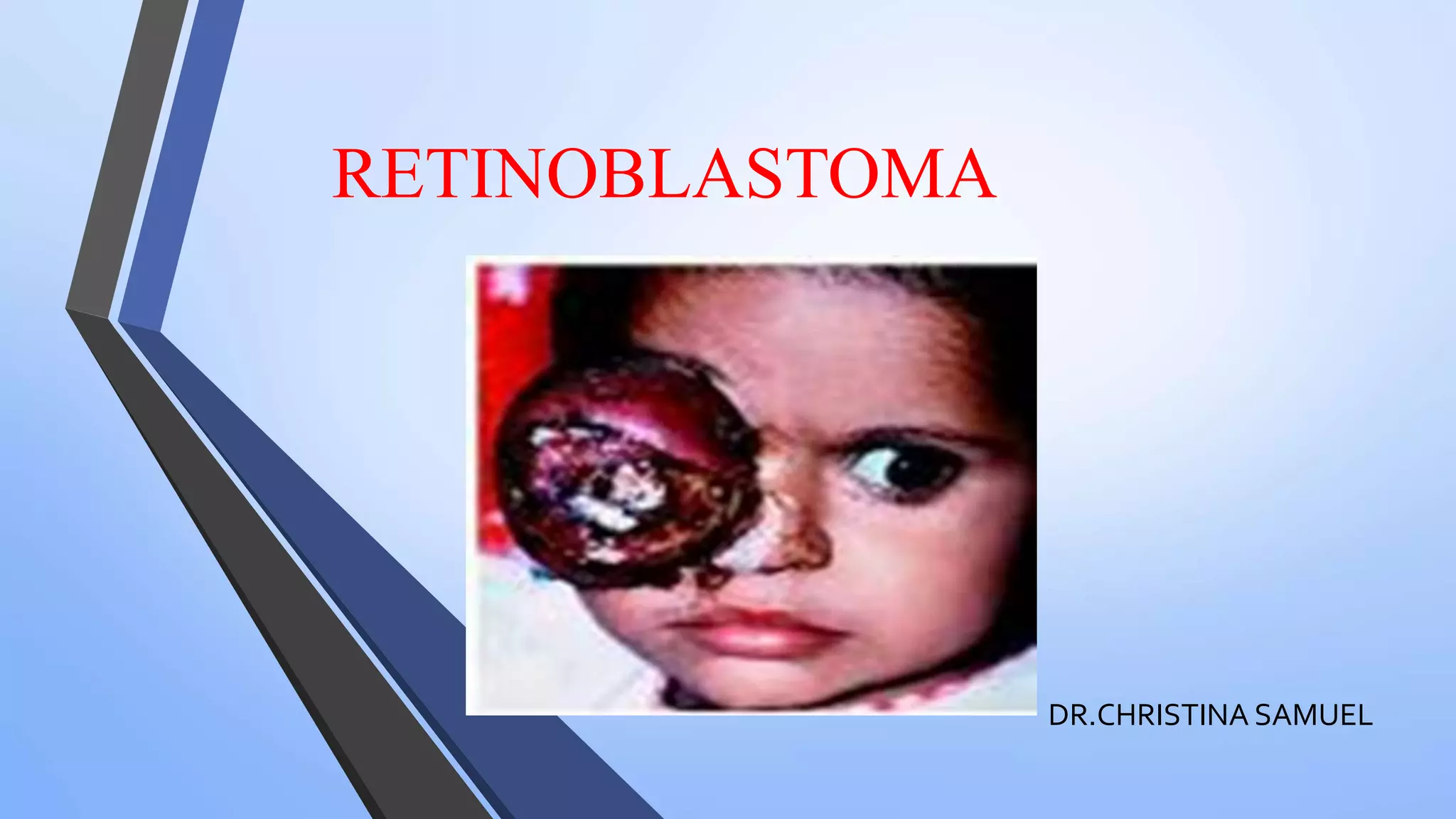
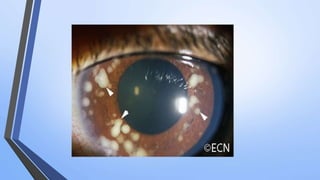
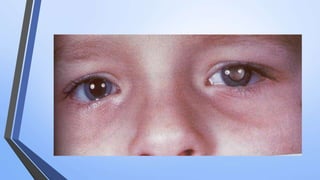
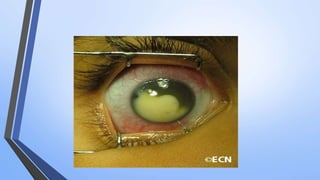
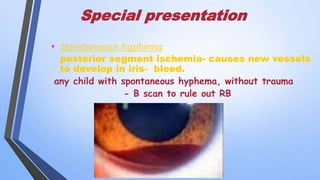
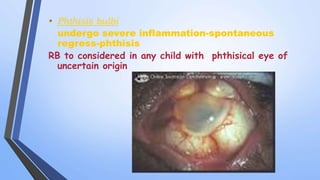
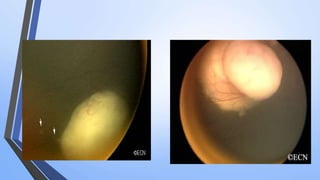
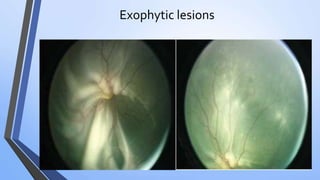
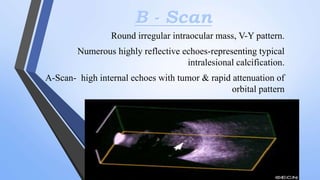
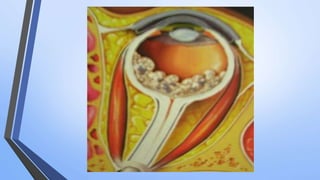
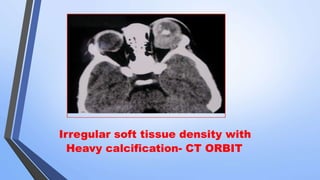
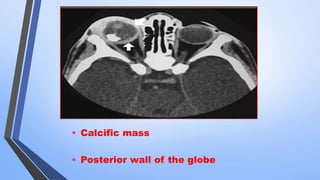
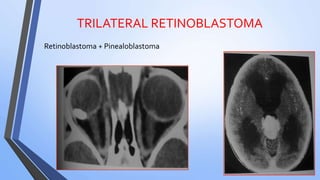
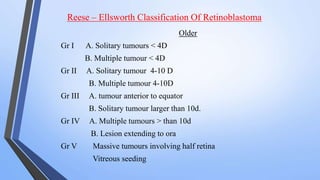
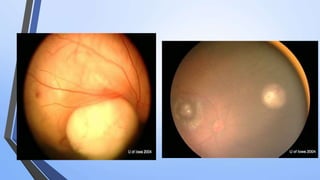
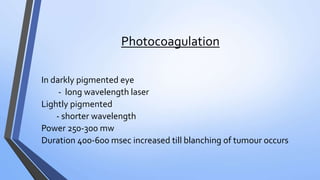
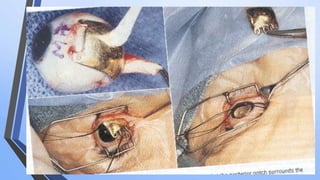
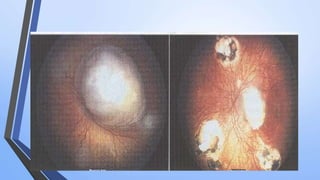
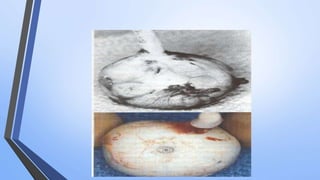
Retinoblastoma is a highly malignant tumor that arises from retinal cells in children. It requires inactivation of both copies of the RB1 tumor suppressor gene. Treatment depends on tumor size and extent, and may include focal treatments like thermotherapy, chemotherapy, brachytherapy or external beam radiation. For more advanced cases, enucleation is needed. Long term follow up is important to monitor for potential recurrence or secondary tumors.