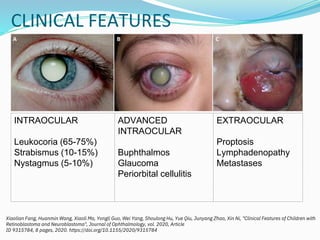
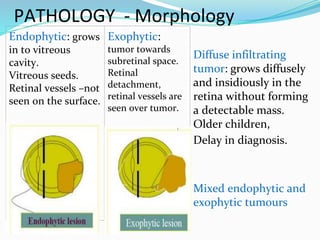
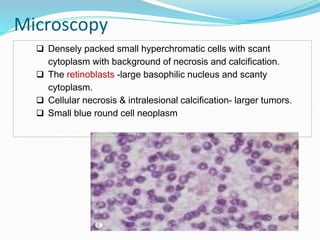
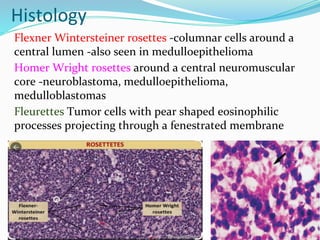
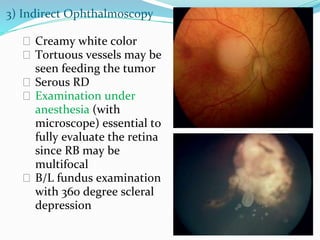
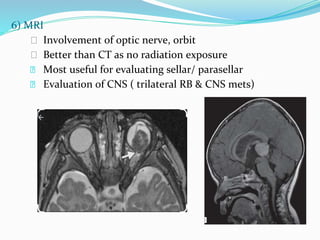
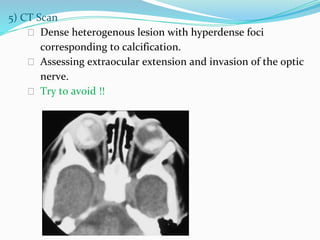
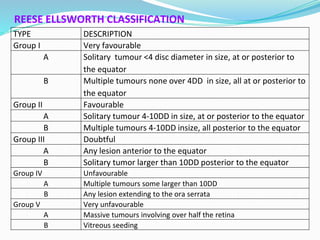
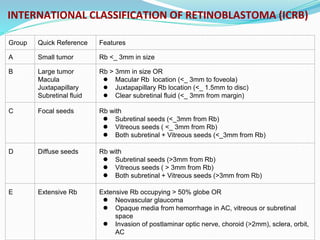
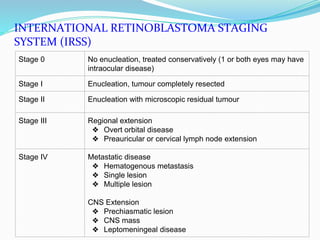
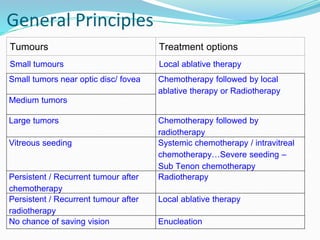
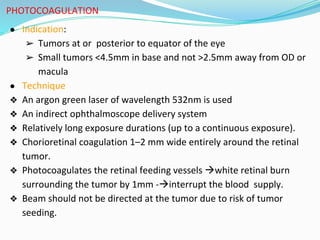
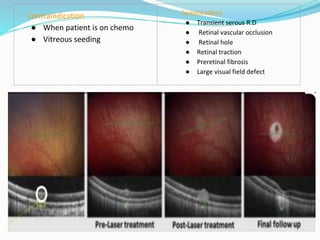
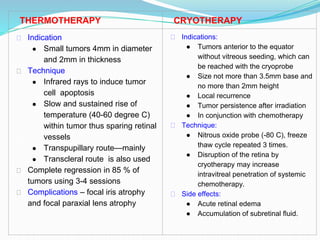
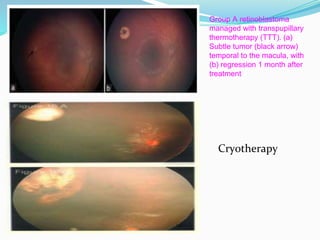
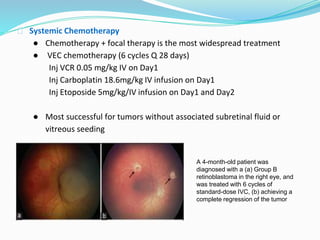
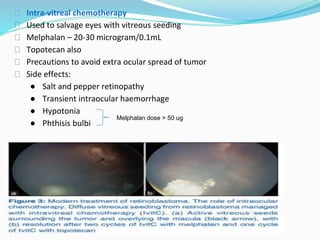
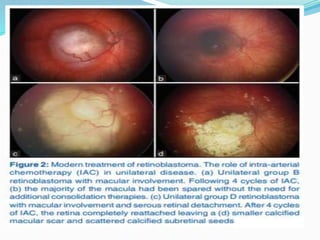
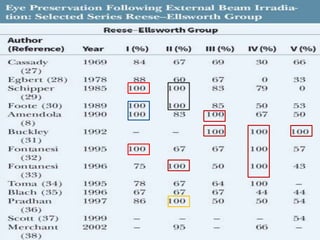
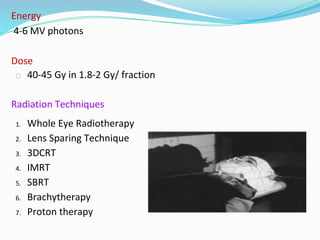
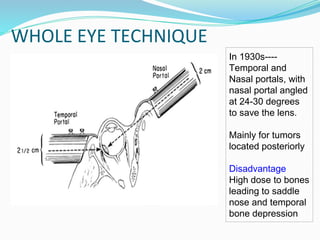
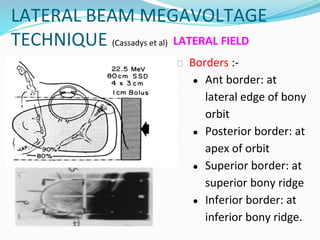
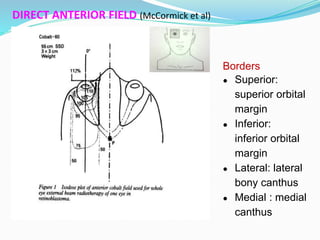
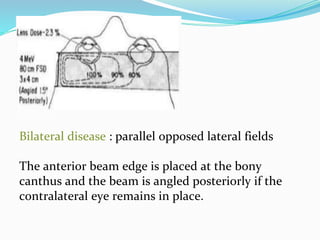
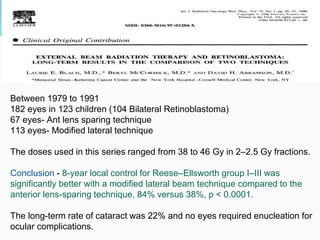
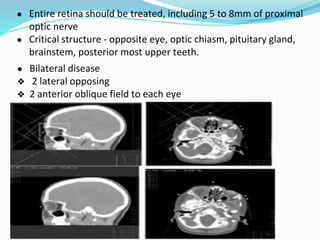
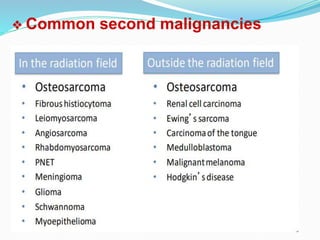
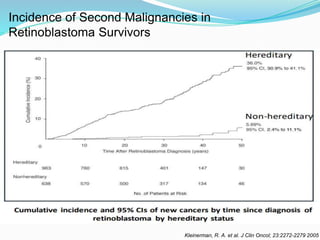
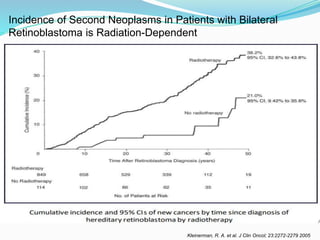
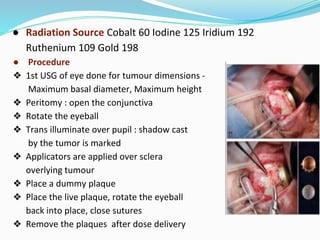
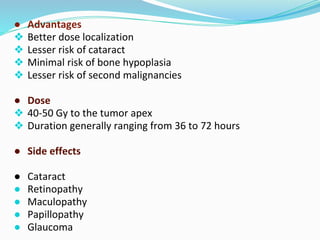
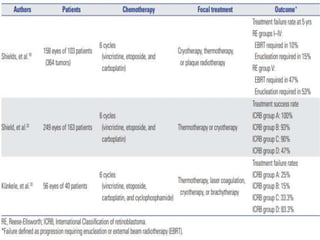
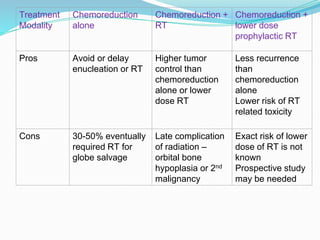
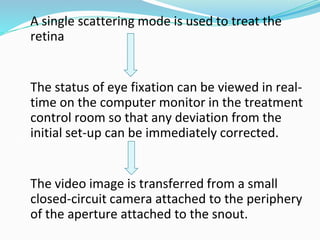
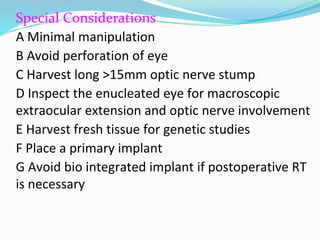
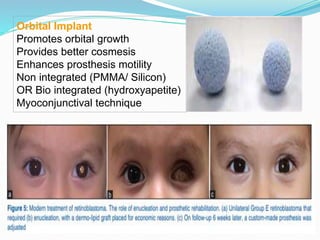
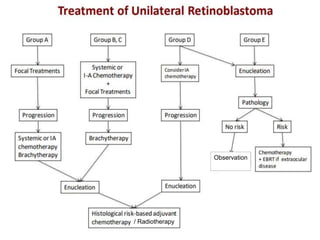
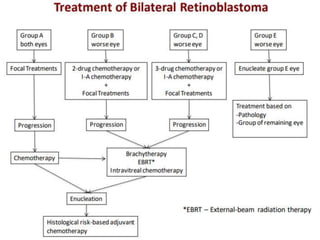
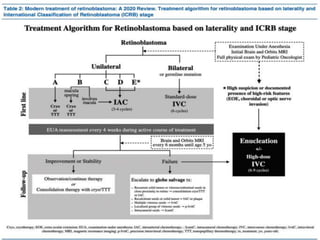
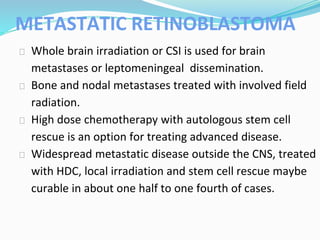
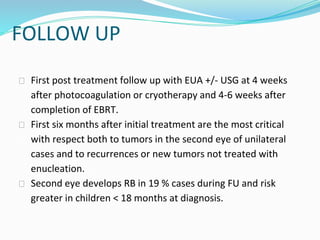
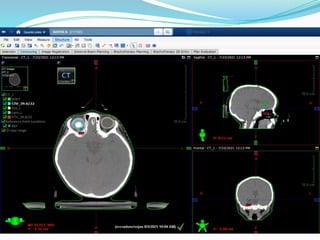
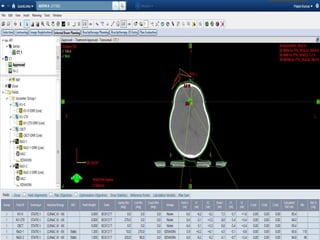
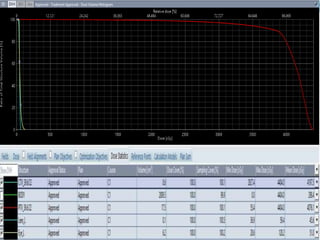
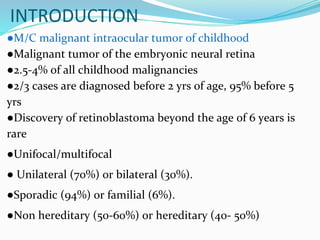
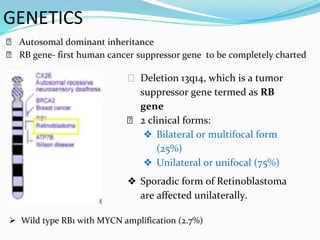
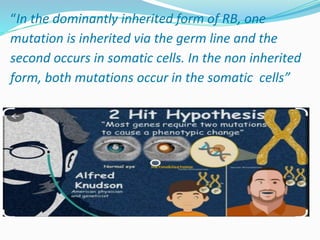
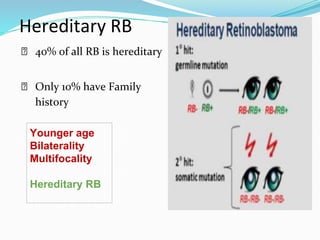
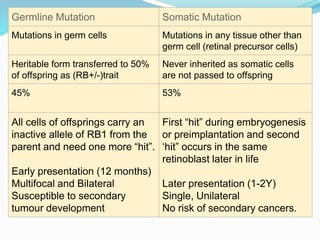
Retinoblastoma is a rare, malignant intraocular tumor of childhood that arises from embryonic retinal cells. It can occur as either a hereditary or non-hereditary form. The hereditary form is caused by a germline mutation in the RB1 gene and accounts for around 40% of cases. It presents at a younger age and is typically bilateral and multifocal. The non-hereditary form occurs due to two somatic mutations and presents later with a unilateral, unifocal tumor. Treatment aims to cure the cancer while preserving vision and the eye depending on the type and stage of disease. Management may involve various local therapies, chemotherapy, and enucleation depending on the classification and extent of involvement.



![Genetic Counselling
➢ Heritable retinoblastoma is inherited in an autosomal
dominant manner.
➢ Individuals with heritable retinoblastoma (H1) have a
heterozygous de novo or inherited germline RB1
pathogenic variant.
➢ Offspring of H1 individuals have a 50% chance of inheriting
the pathogenic variant.
➢ Prenatal testing for pregnancies at increased risk is possible
if the RB1 pathogenic variant has been identified in an
affected family member.
: Lohmann DR, Gallie BL. Retinoblastoma. 2000 Jul 18 [Updated 2018 Nov 21]. In: Adam MP, Ardinger HH, Pagon RA, et al.,
editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021.](https://image.slidesharecdn.com/retinoblastomafinal-220521180634-b60c9f66/85/Retinoblastoma-pptx-10-320.jpg)