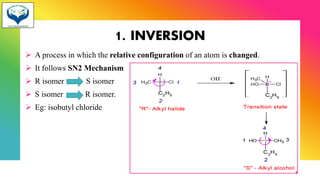
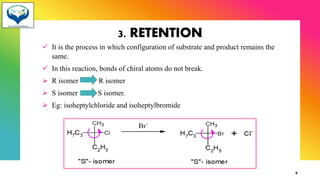
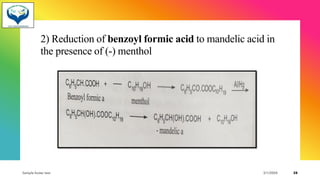
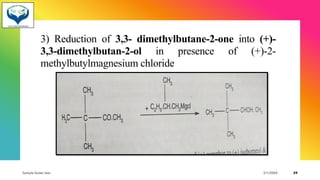
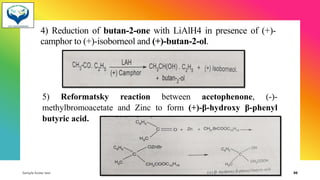
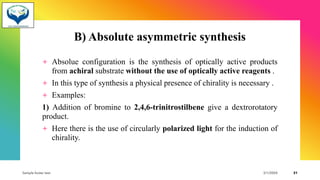
1) Chiral molecules can undergo three types of reactions at the chiral center: inversion, retention, and racemization. Inversion changes the configuration, retention keeps it the same, and racemization converts an enantiomer to a racemic mixture.
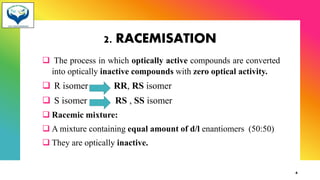
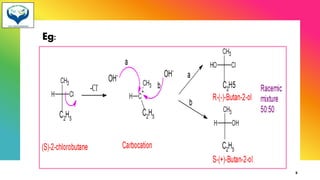
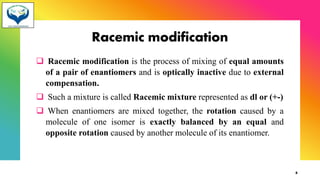
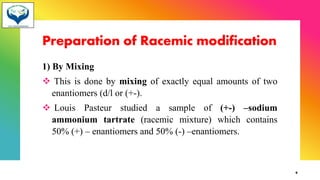
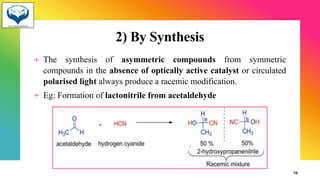
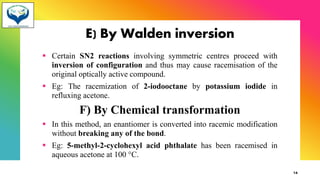
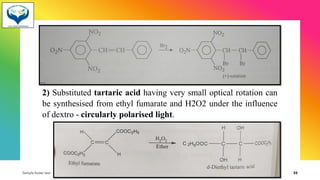
2) Racemization is the process of converting an optically active compound into a racemic mixture. It can occur through mechanisms like anion formation, thermal reactions, or reversible intermediate formation. Racemic mixtures contain equal amounts of both enantiomers and are optically inactive.
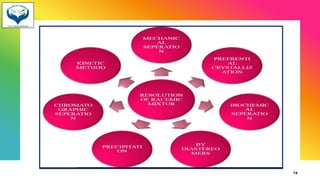
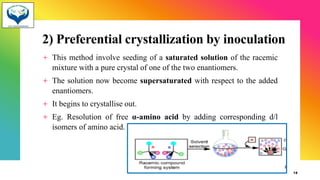
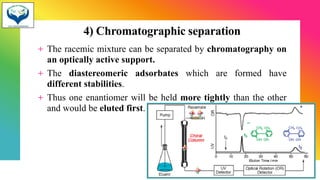
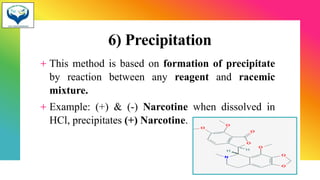
3) Resolution is the separation of a racemic mixture into its pure enantiomers, often by converting them into diastereomers with different properties like crystallization or