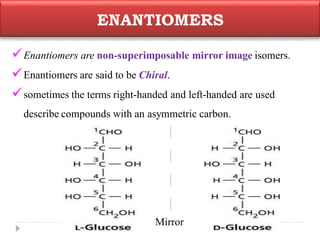
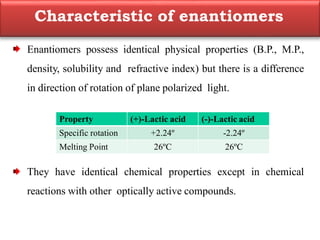
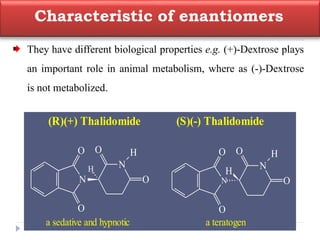
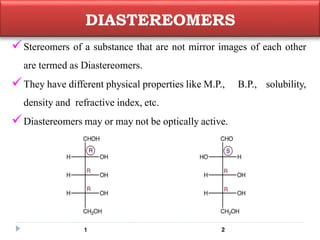
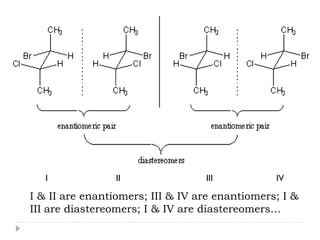
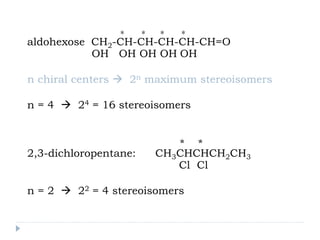
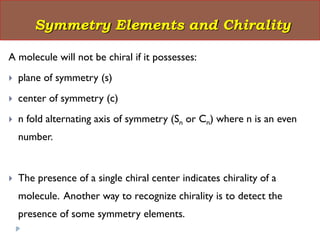
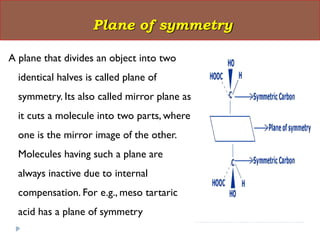
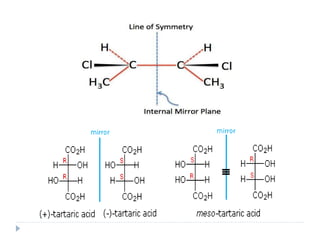
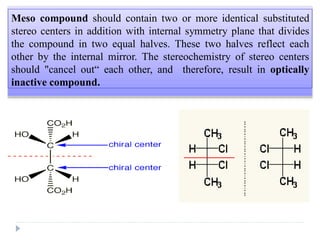
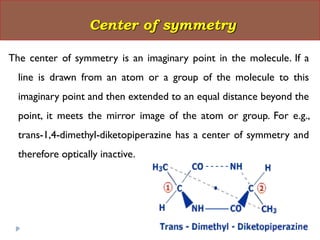
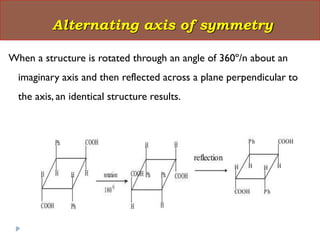
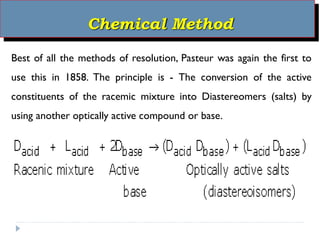
This document summarizes different aspects of stereochemistry, including optical isomers, enantiomers, diastereomers, and methods to separate racemic mixtures. It defines enantiomers as non-superimposable mirror images that have identical chemical and physical properties except when reacting with other chiral compounds. It also describes diastereomers as stereoisomers that are not mirror images and have different physical properties. Common methods for separating racemic mixtures discussed include preferential crystallization, biochemical methods using microorganisms, chemical conversion to diastereomeric salts, and chromatographic techniques.