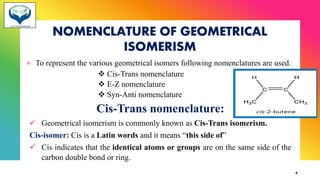
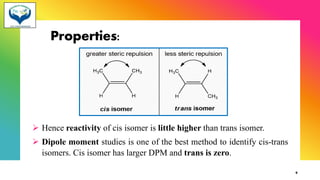
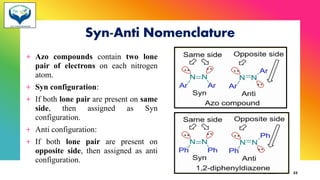
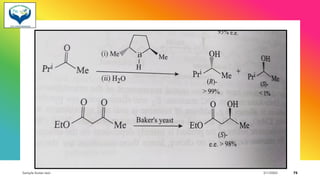
The document provides information about geometrical isomerism including definitions, examples, and methods of determination. It defines geometrical isomerism as arising from restricted rotation around a double bond that leads to different spatial arrangements of atoms. Common types of geometrical isomers include cis-trans, E-Z, and syn-anti. Methods for determining configurations include cyclization reactions, conversion to compounds of known configuration, differences in physical properties, and use of stereoselective or stereospecific reactions.