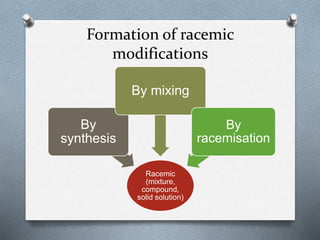
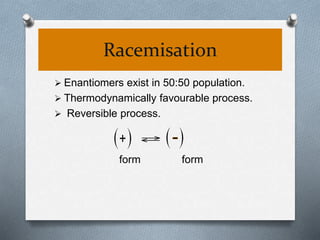
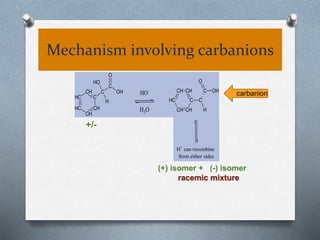
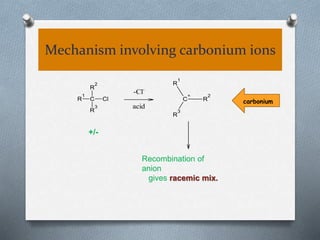
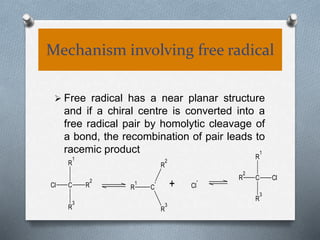
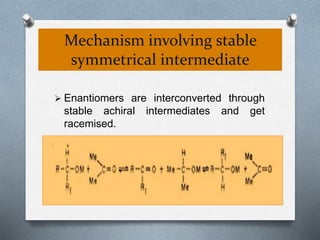
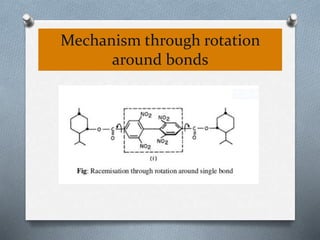
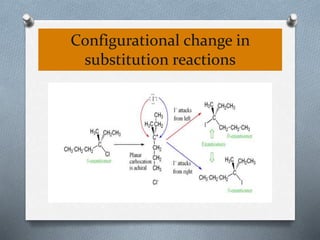
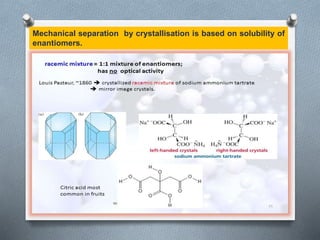
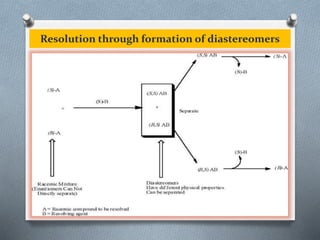
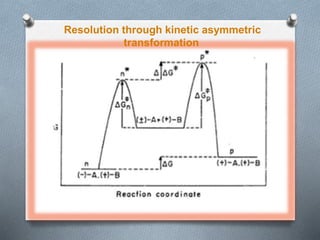
The document discusses racemization and resolution of enantiomers. Racemization is the process where an enantiomer converts to a racemic mixture. Resolution separates the enantiomers of a racemic mixture. Various mechanisms of racemization are described, including those involving carbanions, carbonium ions, free radicals, and stable symmetrical intermediates. Methods of resolution discussed include crystallization, formation of diastereomers, chromatography, equilibrium asymmetric transformations, kinetic asymmetric transformations, biochemical transformations, and inclusion compounds.