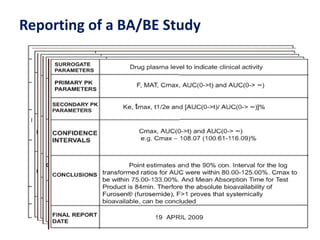
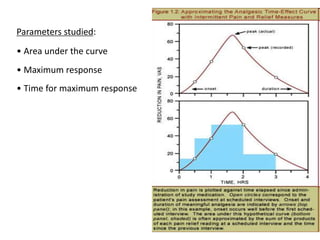
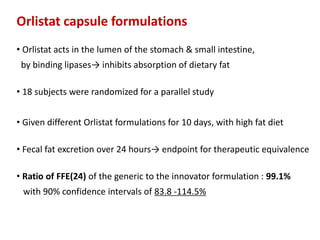
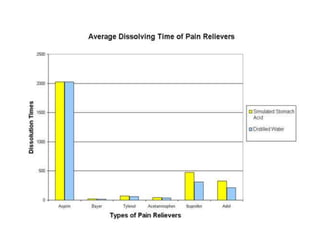
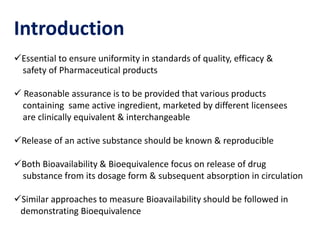
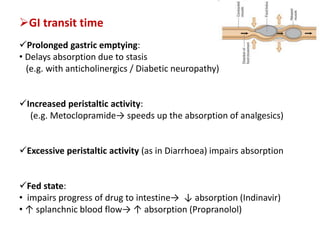
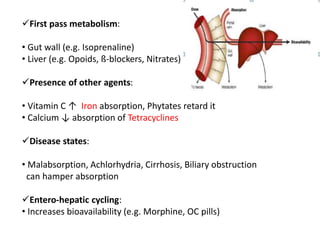
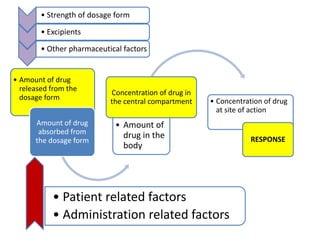
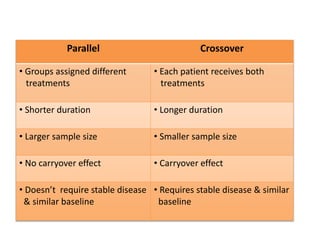
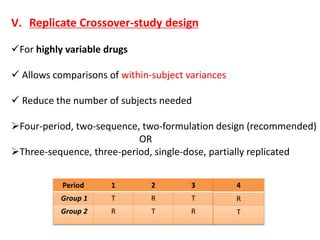
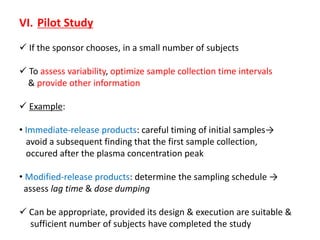
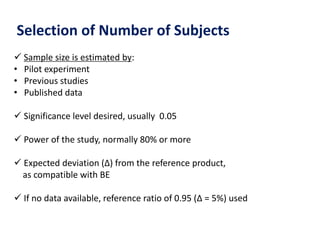
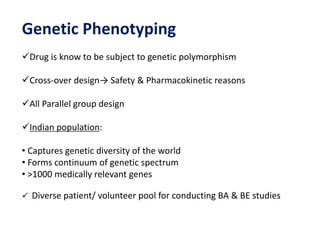
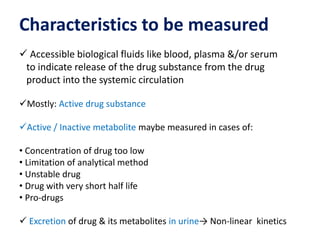
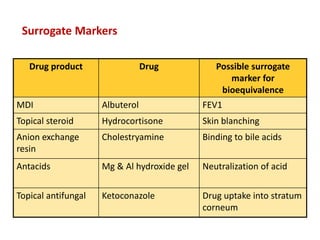
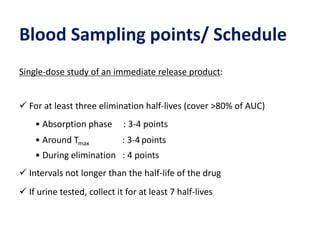
This document discusses bioavailability and bioequivalence studies. It begins by introducing the importance of ensuring uniform quality, efficacy, and safety of pharmaceutical products marketed by different companies. It describes how bioavailability measures the rate and extent that a drug reaches systemic circulation, while bioequivalence ensures drugs from different sources have similar rates and extents of absorption. The document then discusses factors that can affect bioavailability like dosage form, physiology, and disease states. It also defines pharmaceutical equivalents and alternatives in establishing bioequivalence. Finally, it provides an overview of the different study designs used in pharmacokinetic studies to assess bioequivalence.


![Factors affecting Bioavailability of a Drug
Physical properties of a drug
Physical state:
• Liquids > Solids
[ Solution > Suspension > Capsule > Tablet > Coated tablet ]
• Crystalloids > Colloids
Lipid or water solubility:
• Aqueous phase at absorption site
• Passage across Cell surface](https://image.slidesharecdn.com/9-151210145329/85/ba-be-studies-4-320.jpg)























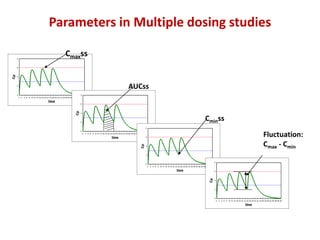

![Statistical Evaluation
Primary concern of bioequivalence is to limit Consumer’s &
Manufacturer’s risk
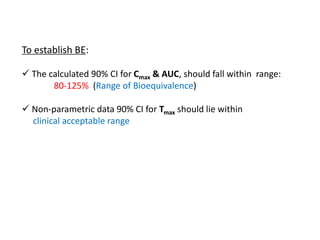
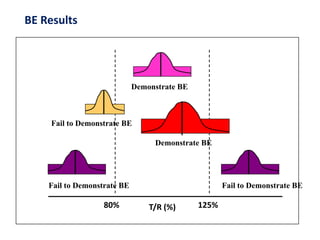
• Cmax & AUC analysed using ANOVA
• Tmax analysed by non-parametric methods
Use natural log transformation of Cmax and AUC
• Calculate Geometric means of Cmax of Test [Cmax’t]
• Calculate Geometric means of Cmax of Reference [Cmax’r]
• Calculate Geometric Mean Ratio= [Cmax’t] / [Cmax’r]
Calculate 90% confidence interval for this GMR for Cmax
Similarly calculate GMR for AUC](https://image.slidesharecdn.com/9-151210145329/85/ba-be-studies-53-320.jpg)





![ Volunteer Selection & Recruitment
Volunteers called 1 day before study & admitted
Written ICF taken
During the Study
Standardized study environment
Vital signs examination at scheduled times
Standardised amount of water [~240ml]
No concomitant medications [including herbal remedies]](https://image.slidesharecdn.com/9-151210145329/85/ba-be-studies-65-320.jpg)



![Maintenance of Records & Retention of
Study Samples
All Records of in vivo tests on any marketed batch of a
drug product should be maintained by the Sponsor
for atleast 2 years after expiry date of the batch
All Drug samples to be retained for a period of atleast
3 years after conduct of the study
OR
1year after expiry of the batch
[Stored in conditions consistent with the product labeling]](https://image.slidesharecdn.com/9-151210145329/85/ba-be-studies-69-320.jpg)