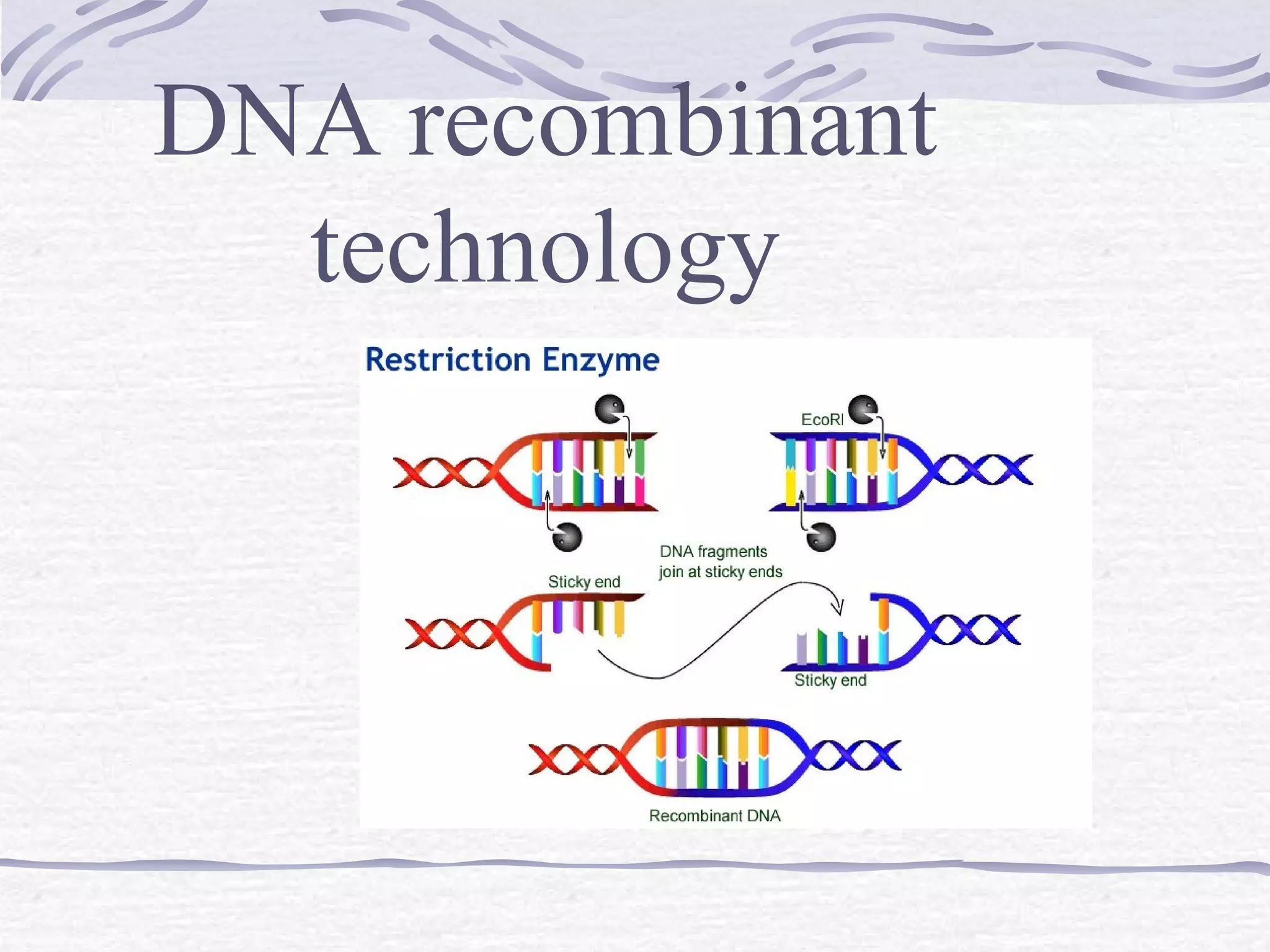
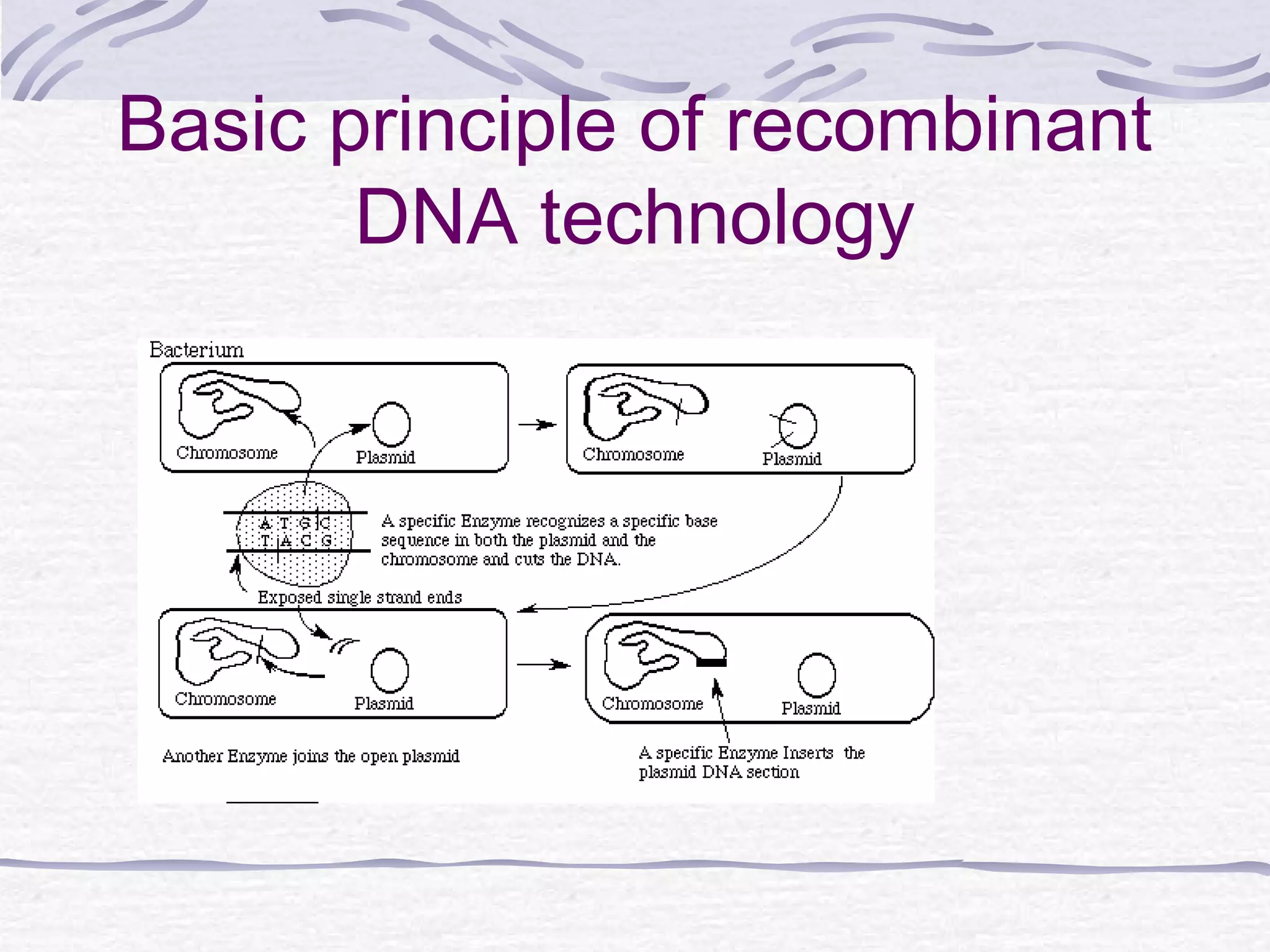
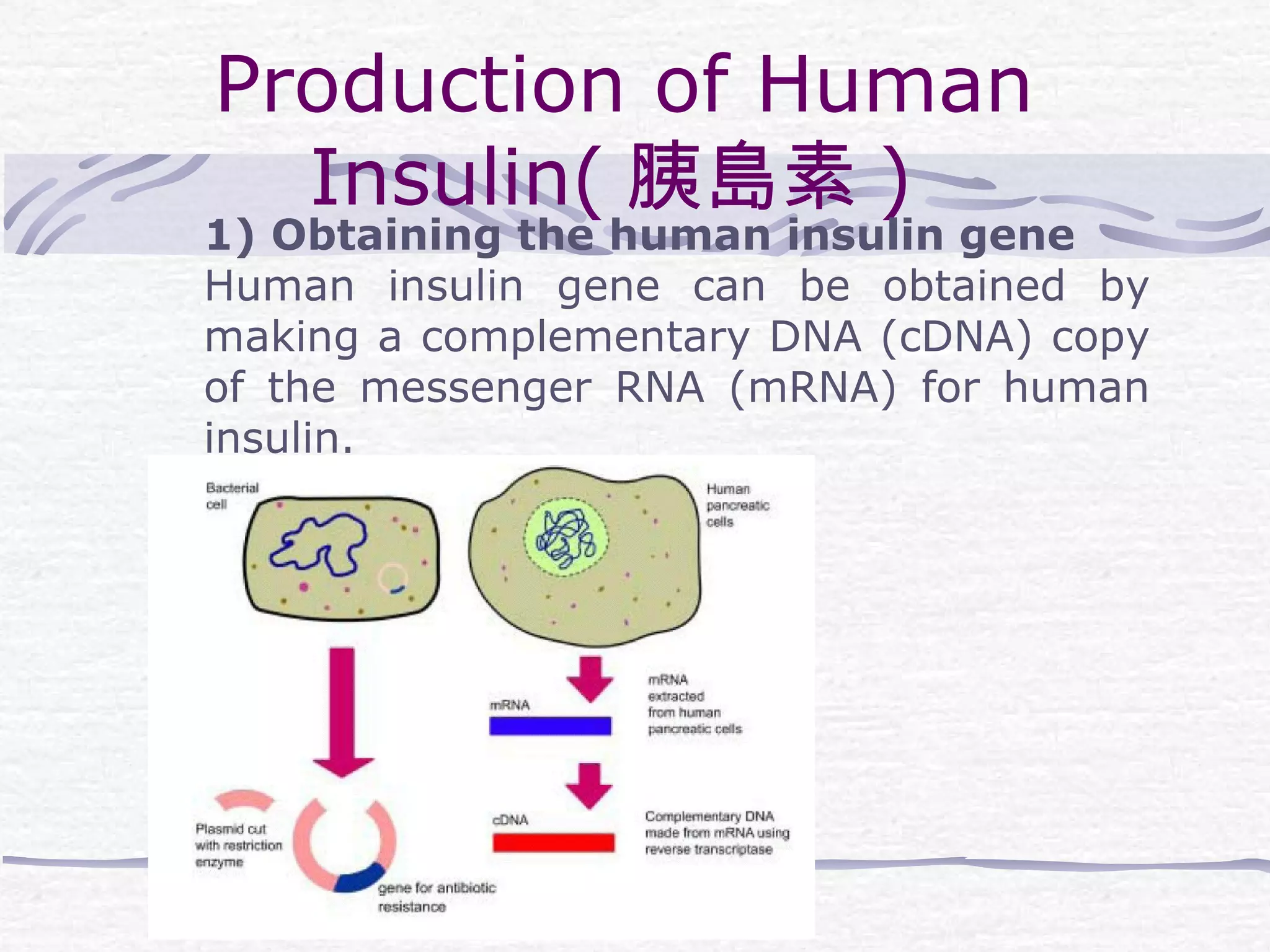
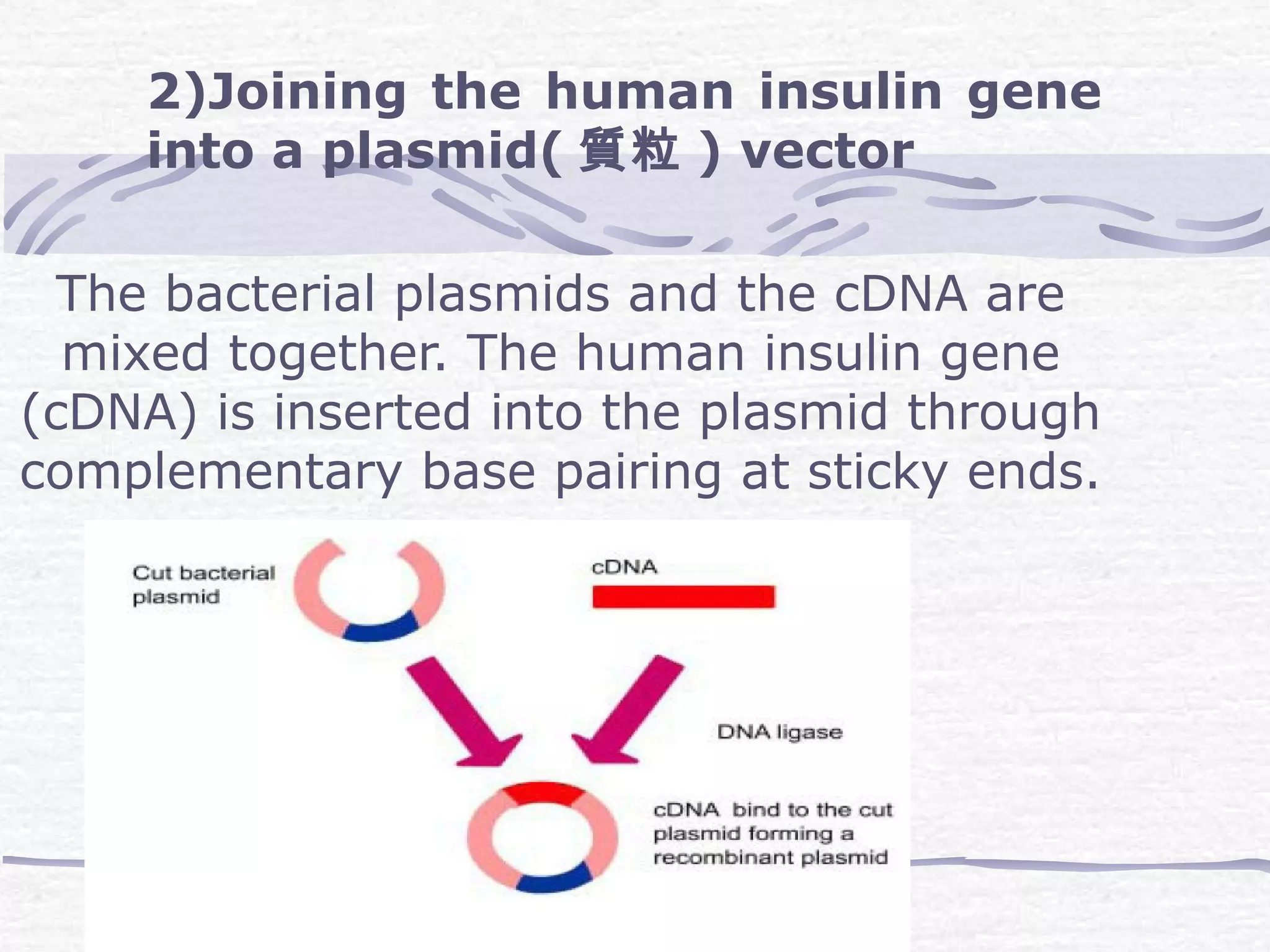
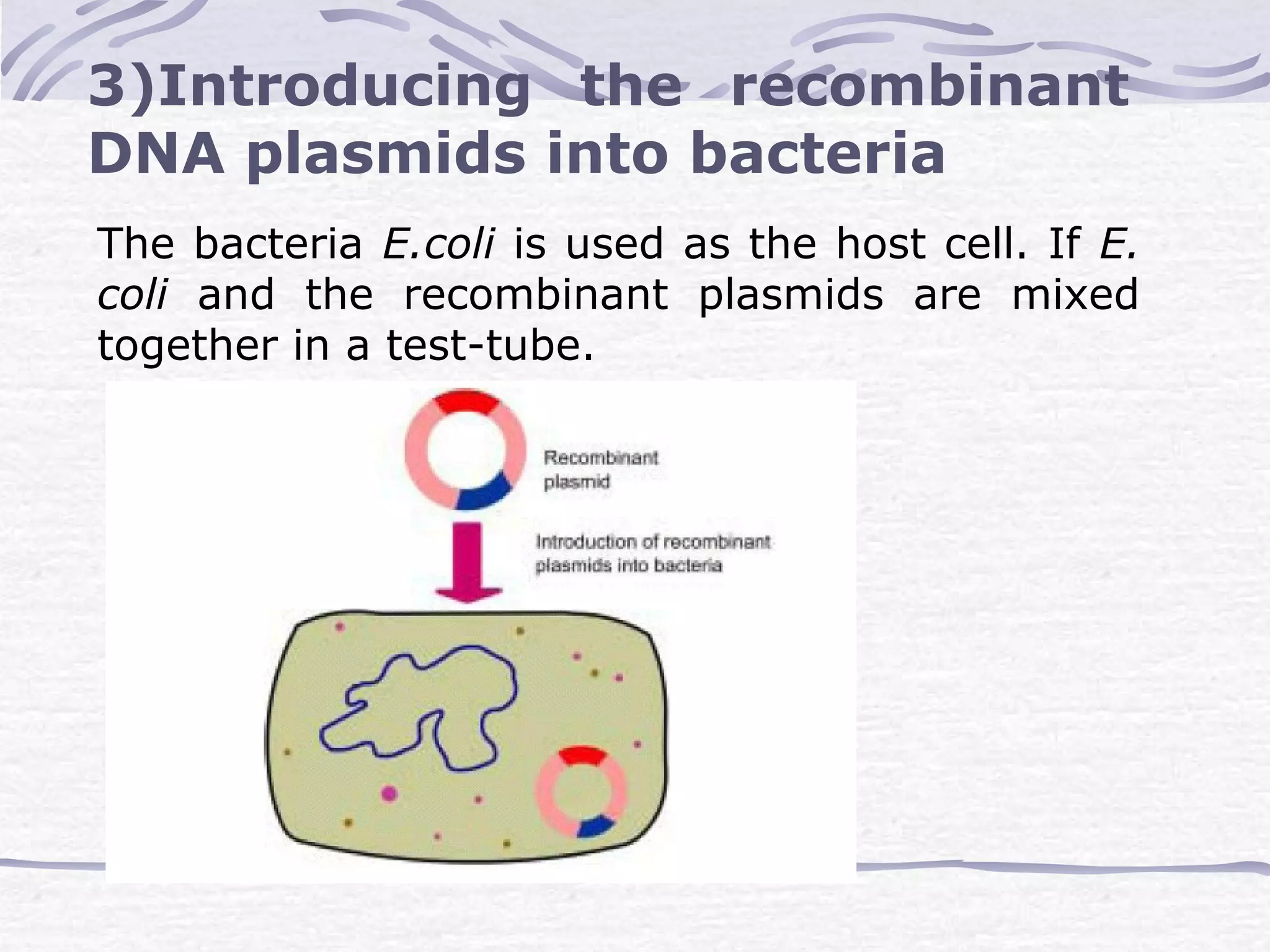
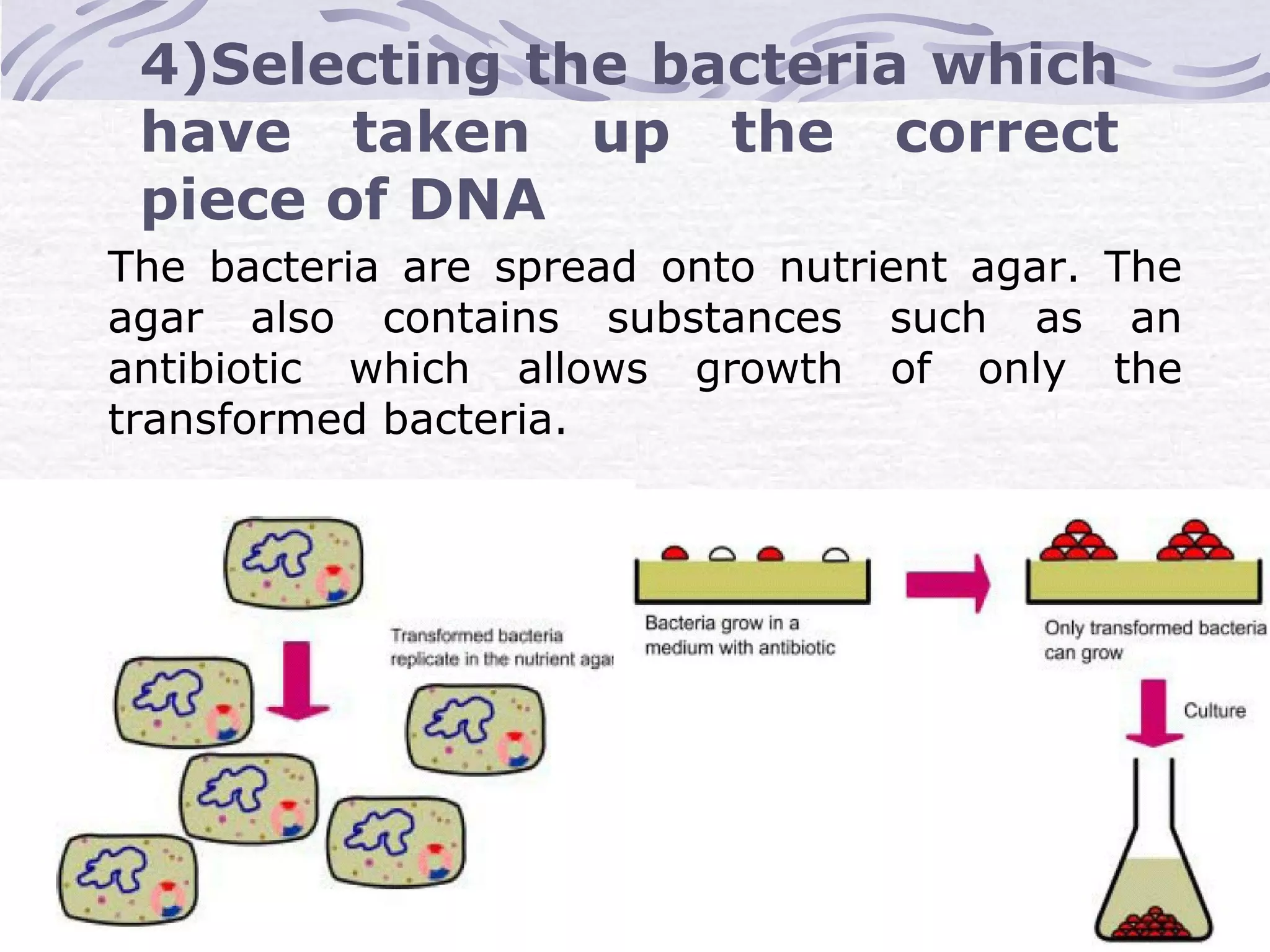
Recombinant DNA technology involves recombining DNA segments and allowing recombinant DNA molecules to enter cells and replicate. It was developed in 1973 by scientists Boyer and Cohen. The basic principle is to insert DNA into a vector, introduce it into a host cell where it replicates and produces the gene. Applications include producing human proteins like insulin through genetically engineered bacteria. Safety issues involve ensuring recombinant bacteria do not escape the laboratory and cause epidemics, which is addressed through physical and biological containment methods overseen by regulatory committees.