Embed presentation
Download as PDF, PPTX


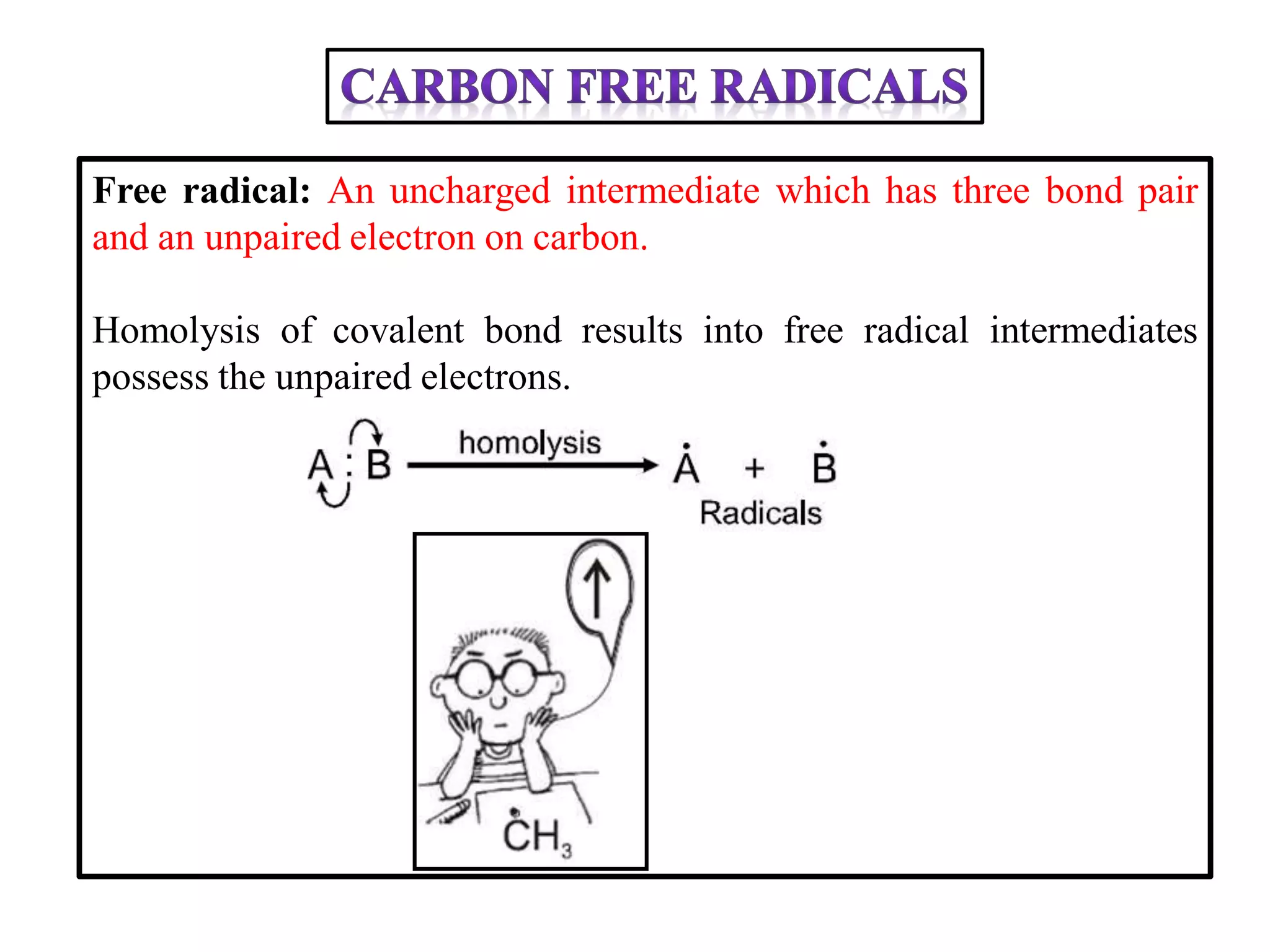

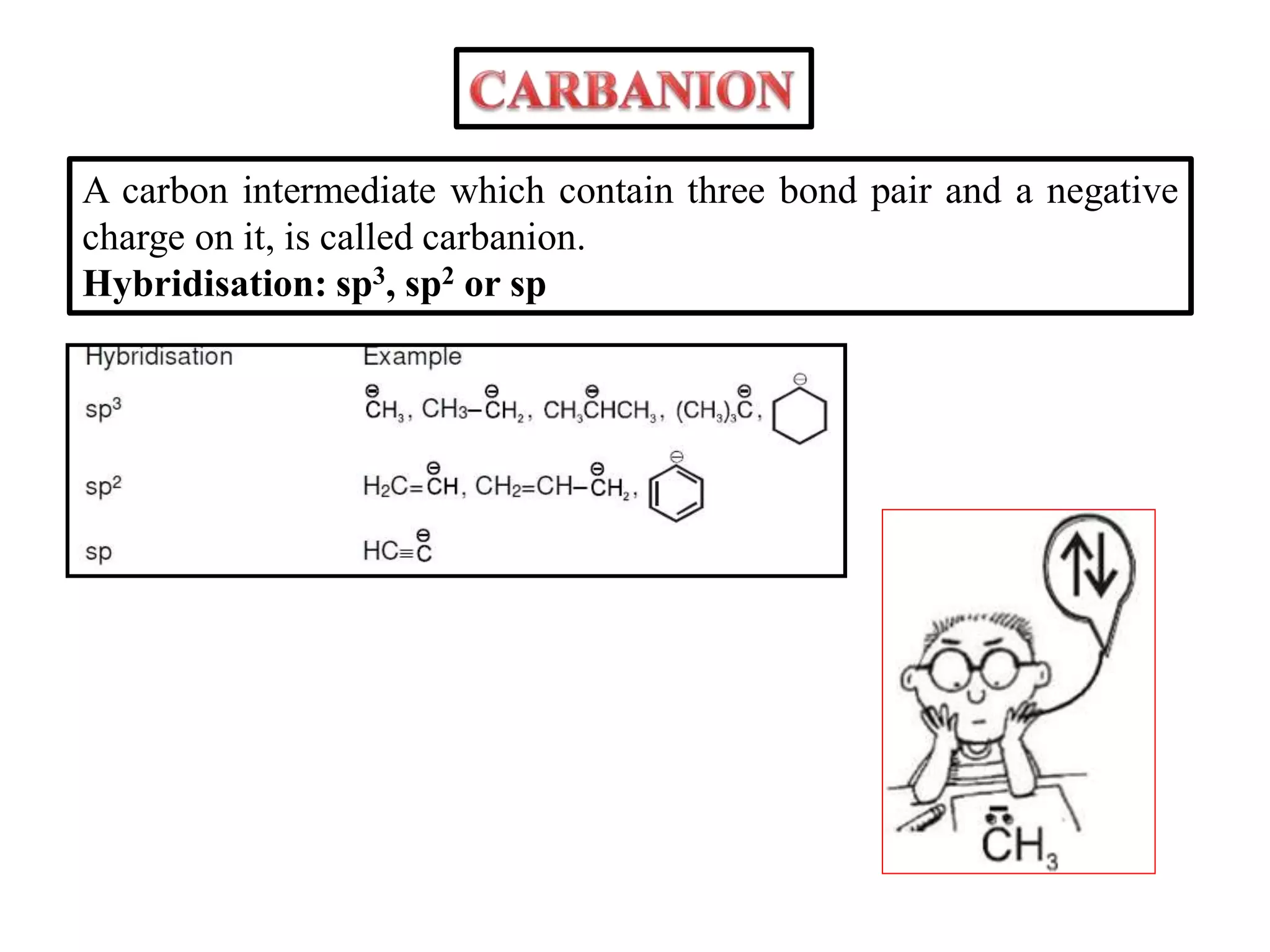
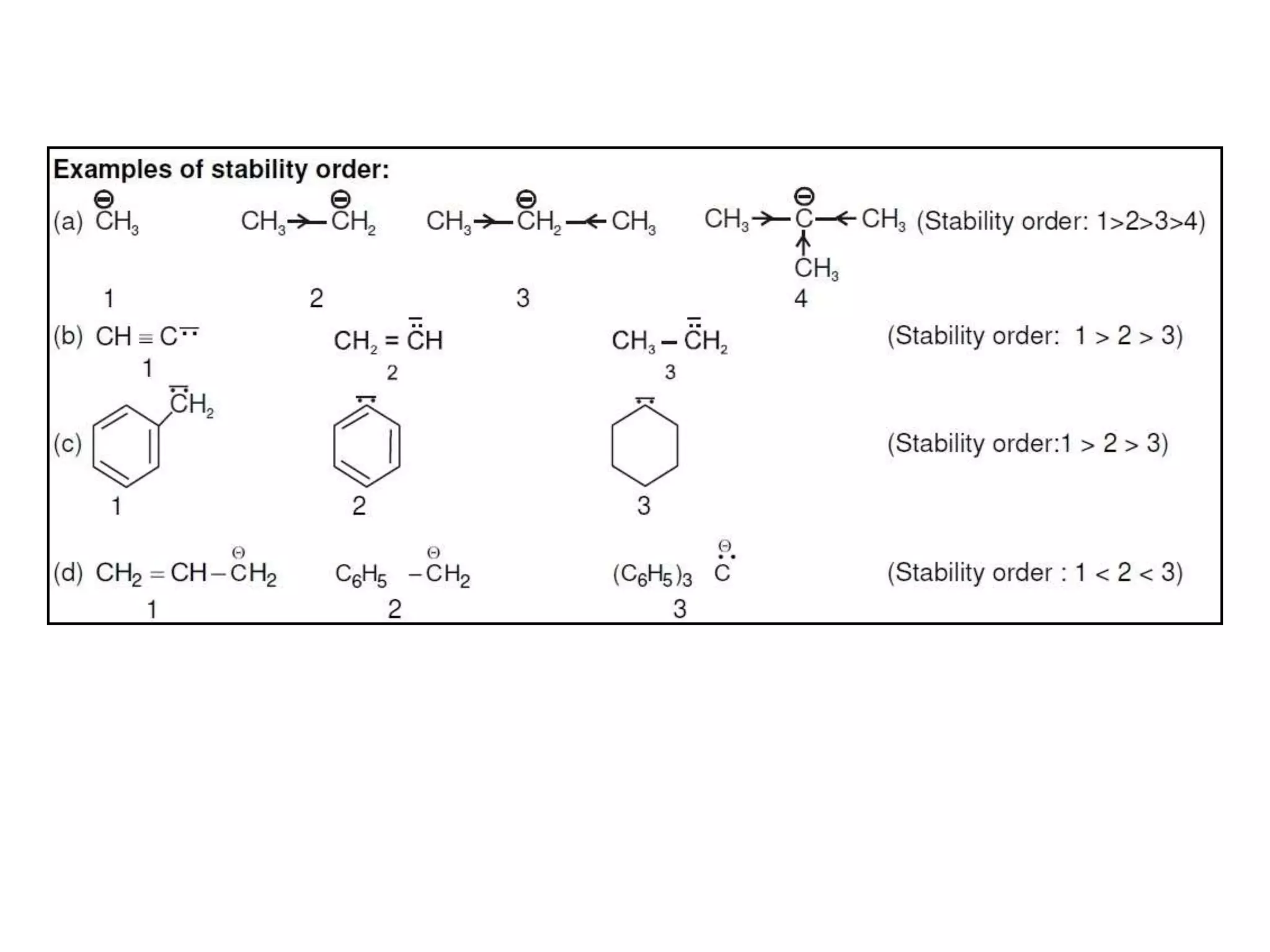
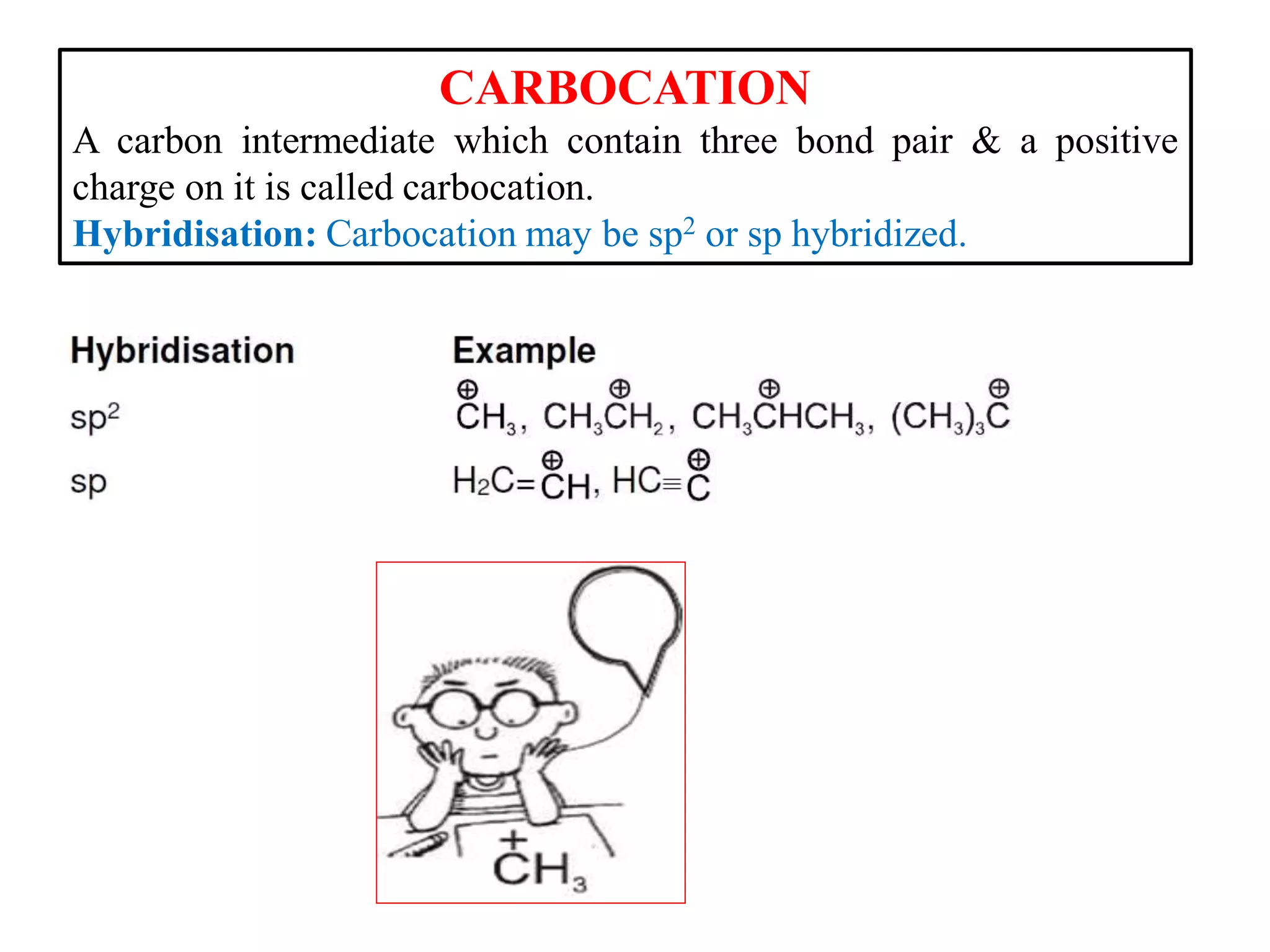

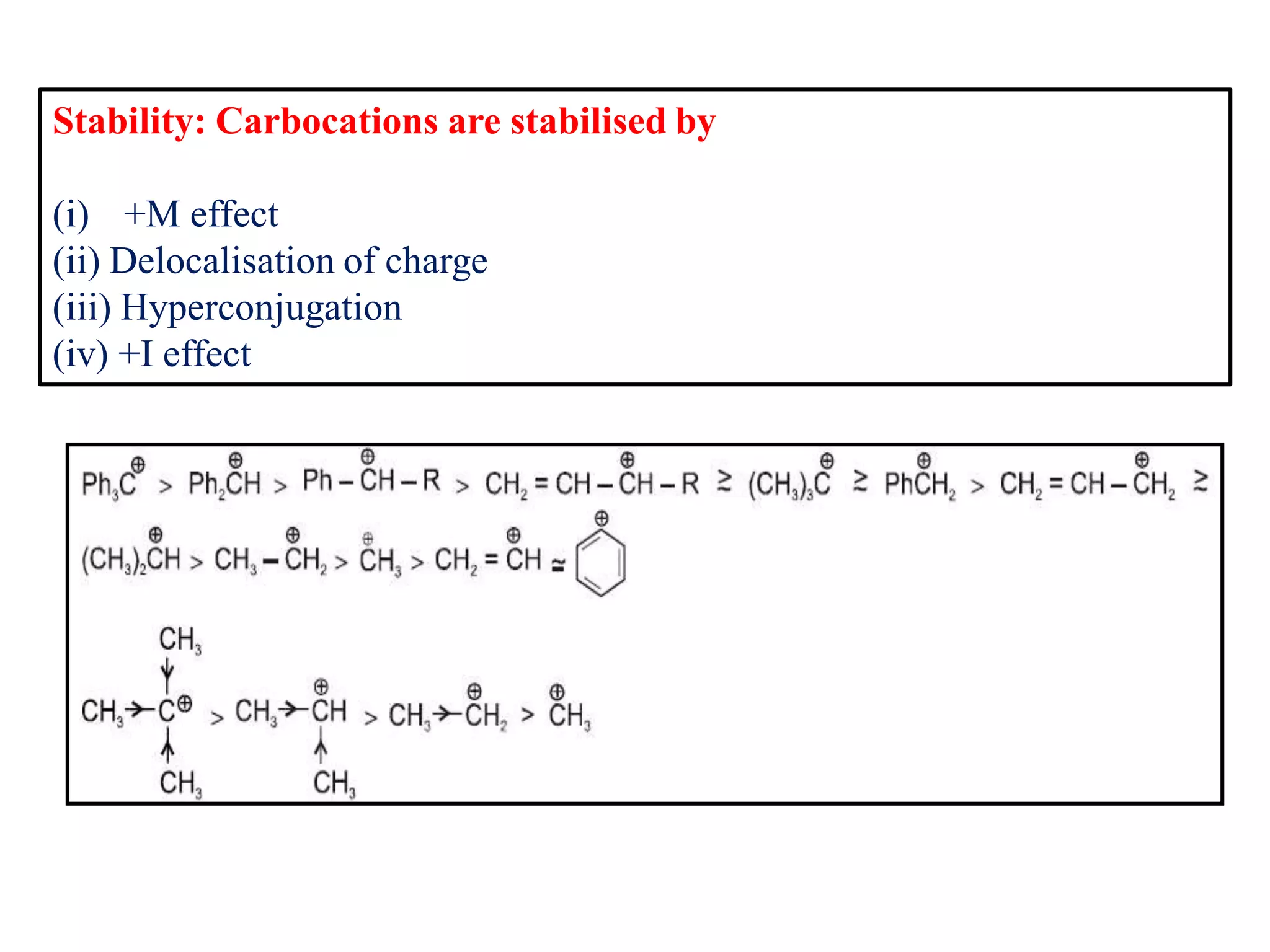
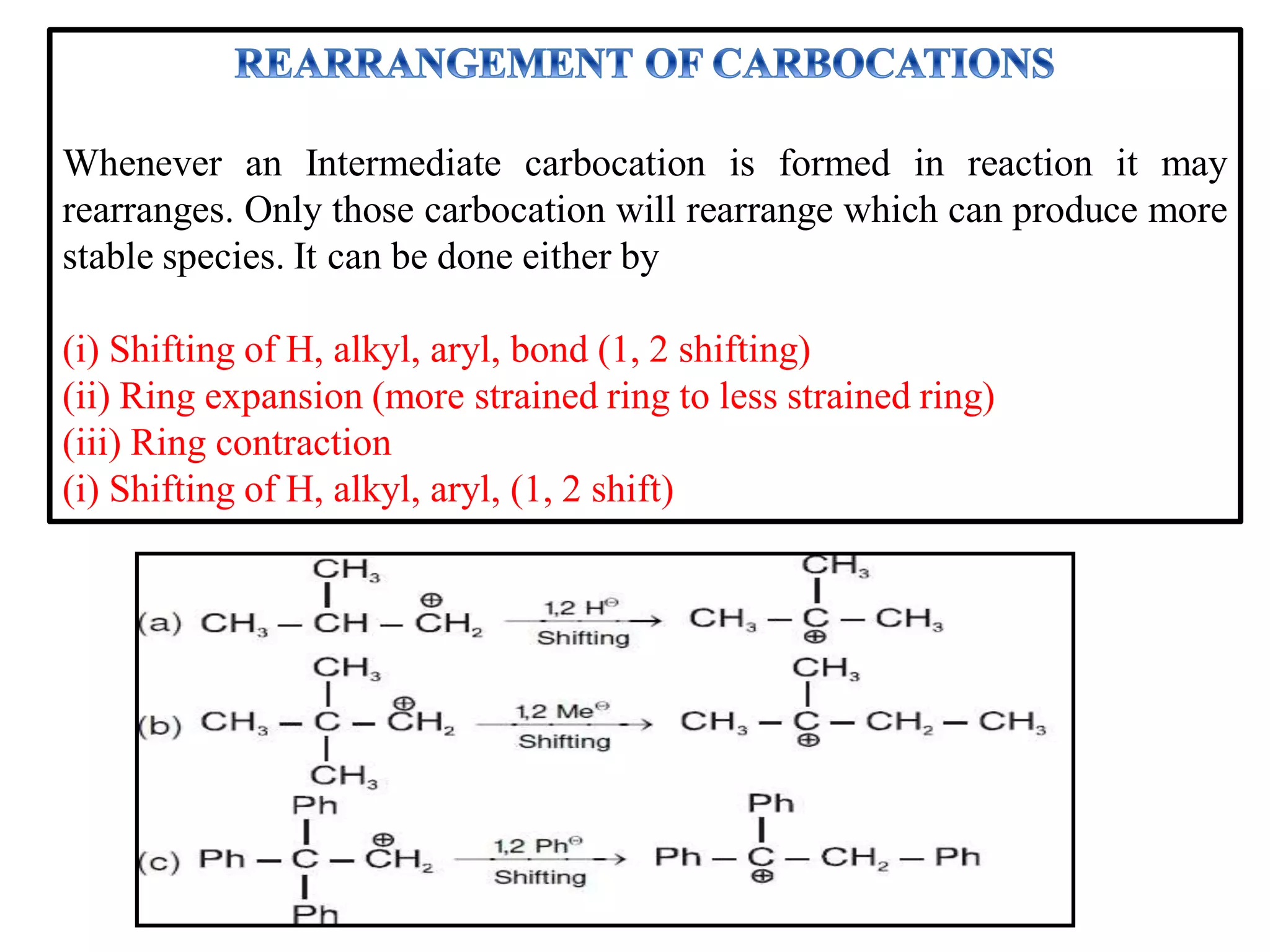

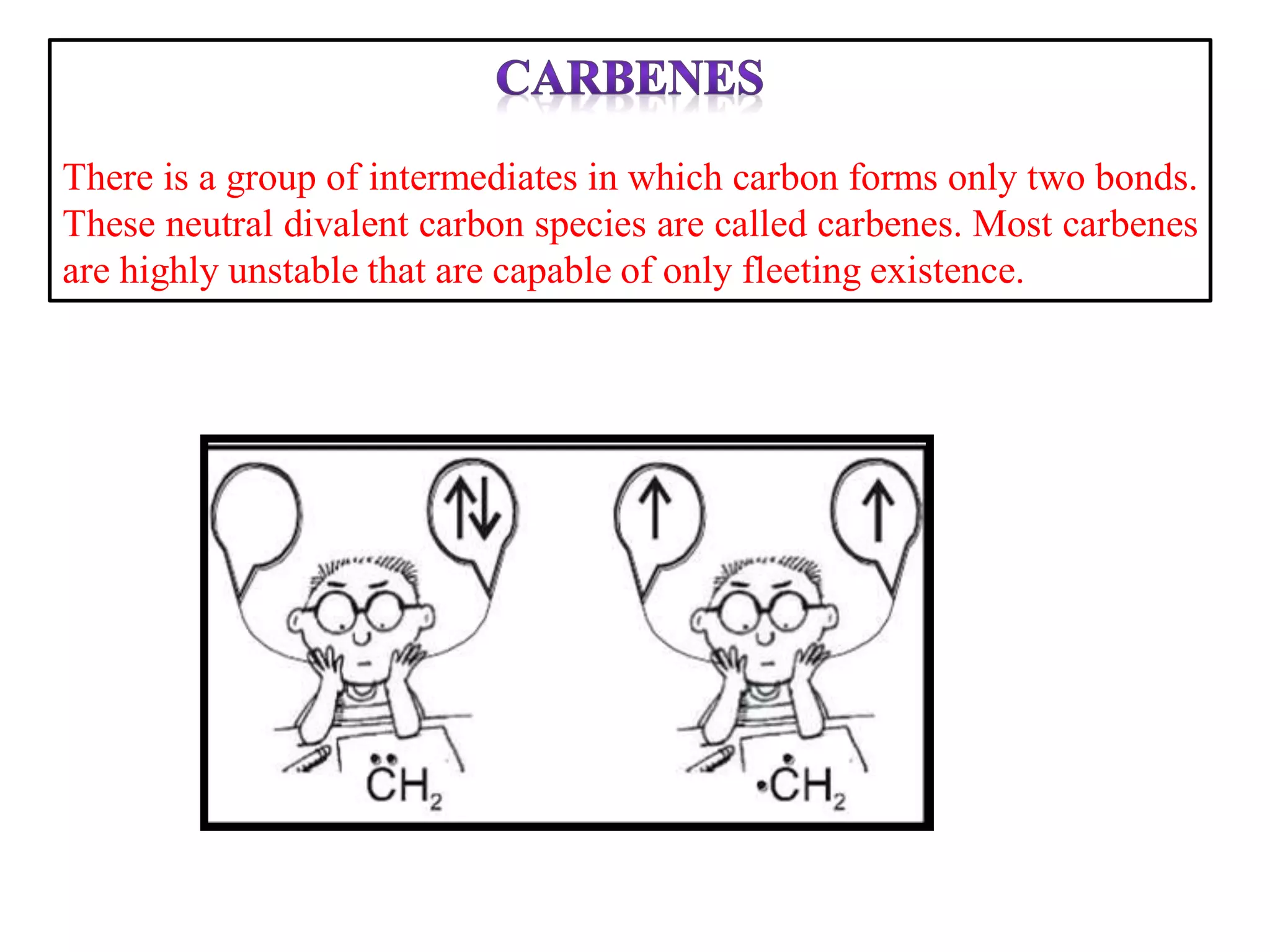

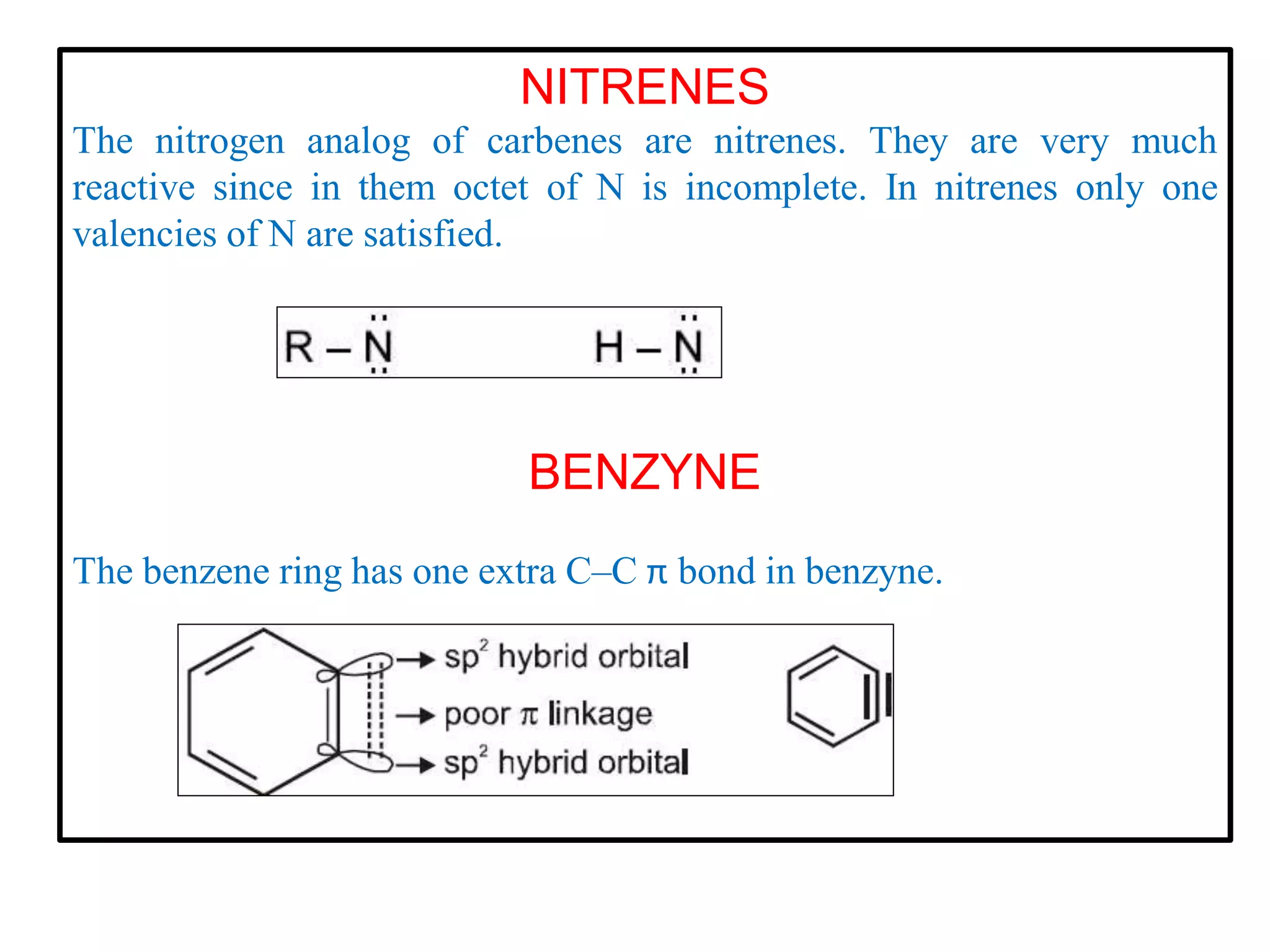

This document discusses various reactive intermediates in organic chemistry including free radicals, carbanions, carbocations, carbenes, and nitrenes. It notes that free radicals contain an unpaired electron and are generated by photolysis, heat, or peroxides. Carbocations are carbon intermediates with a positive charge that are stabilized through effects like resonance, hyperconjugation, or induction. They can rearrange through 1,2 shifts or ring expansions/contractions to form more stable structures. Carbenes are neutral divalent carbons that exist fleetingly, while nitrenes contain an incomplete octet on nitrogen, making them highly reactive.














