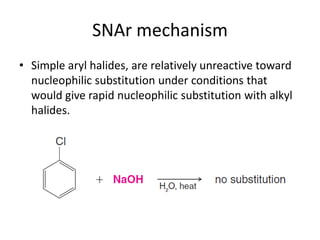
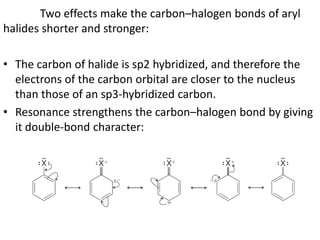
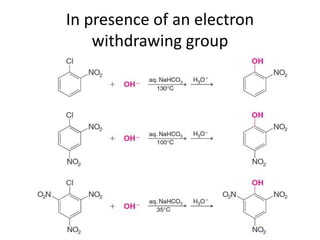
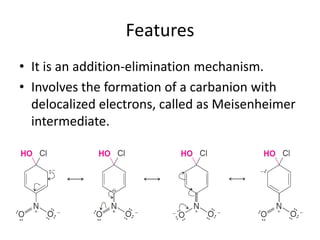
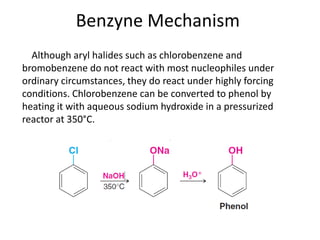
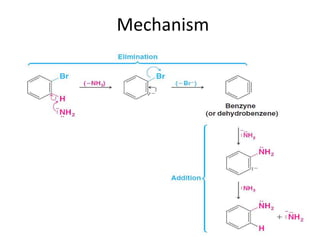
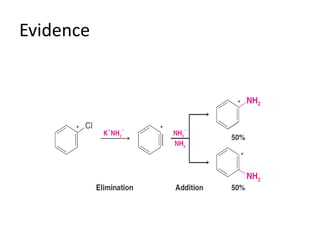
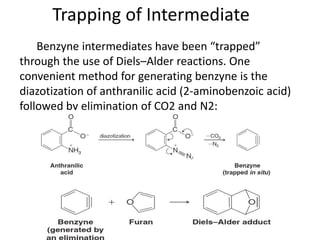
This document summarizes the aromatic nucleophilic substitution (SNAr) reaction mechanism. It involves the formation of a carbanion intermediate called the Meisenheimer intermediate through an addition-elimination process. Aryl halides are relatively unreactive toward nucleophilic substitution, but reactivity increases in the presence of electron-withdrawing groups due to stabilization of the carbanion. Under highly forcing conditions, aryl halides can undergo substitution through a benzyne intermediate that has been trapped using Diels-Alder reactions.