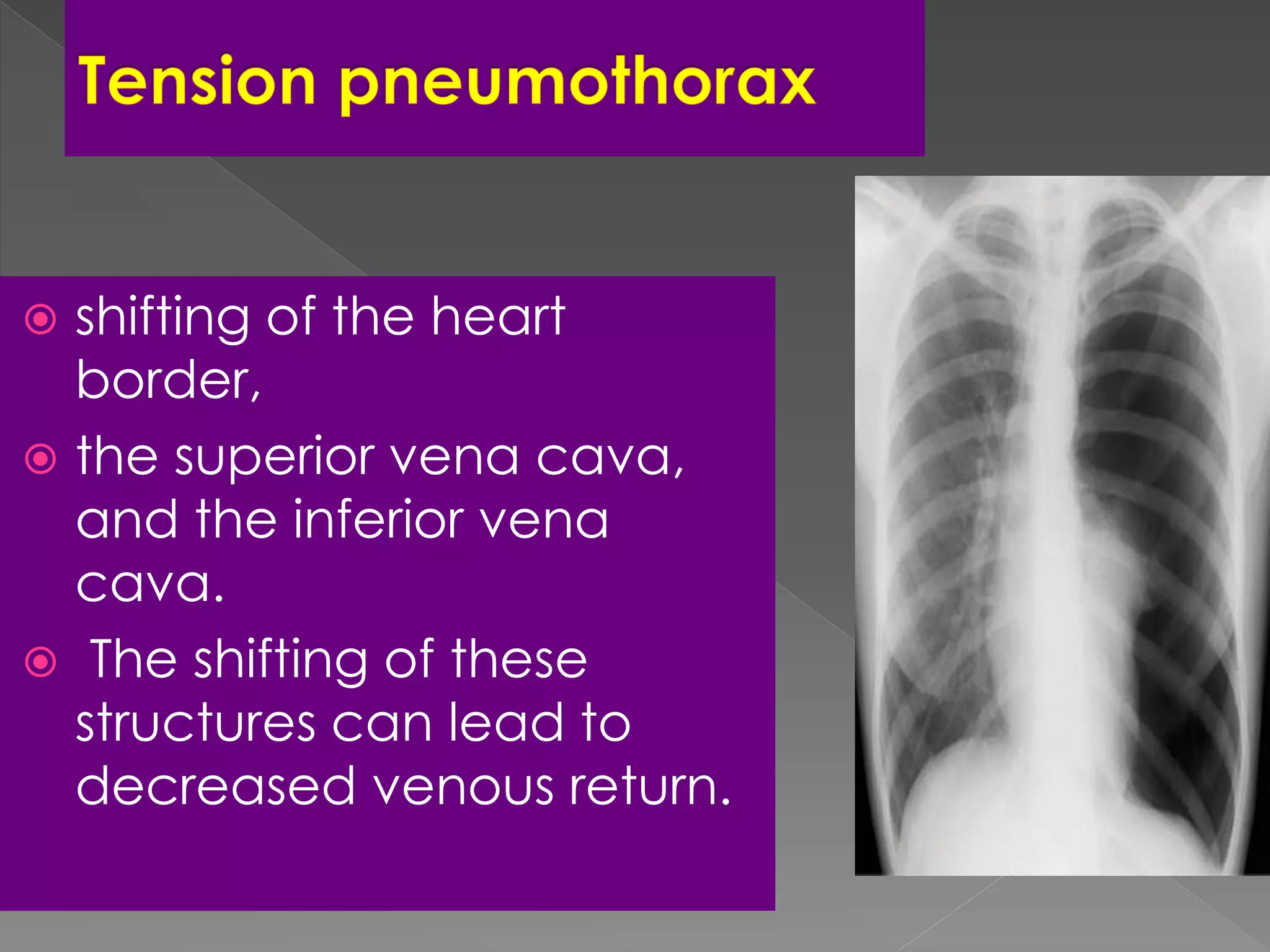
This document discusses various radiographic signs seen on chest x-rays and CT scans related to different types of lung collapse and cystic lung lesions. It describes signs such as the flat waist sign seen in left lower lobe collapse, the juxtaphrenic peak sign seen in upper lobe collapse, and the fallen lung sign seen with bronchial fractures. It also discusses cystic lung patterns seen in conditions like lymphangioleiomyomatosis (LAM), Langerhans cell histiocytosis, and lymphocytic interstitial pneumonia. Different characteristics of cysts such as their size, distribution and appearance on imaging are described for these various conditions.