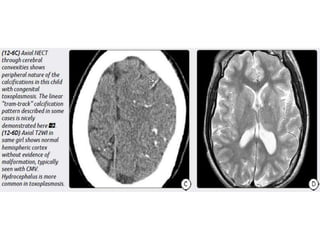
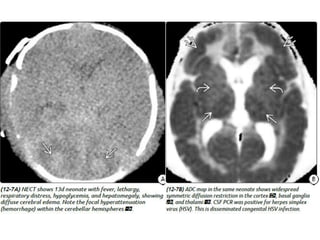
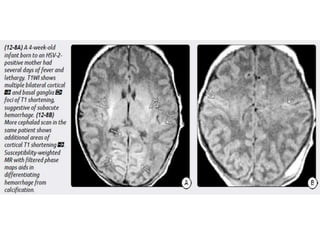
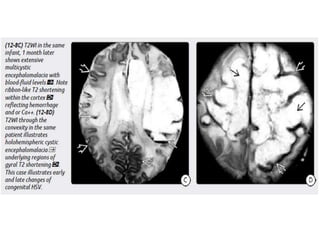
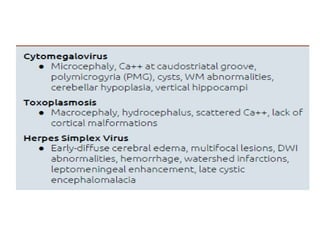
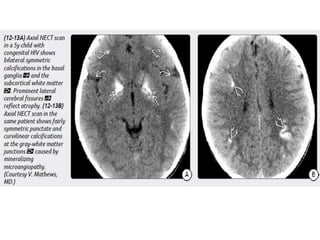
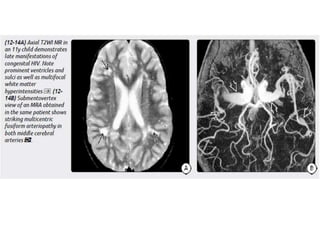
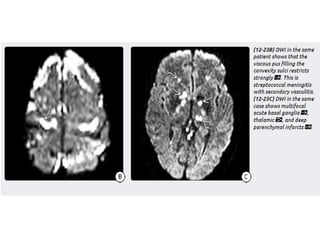
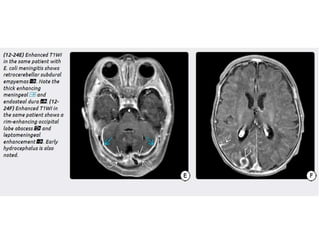
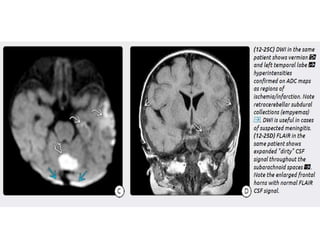
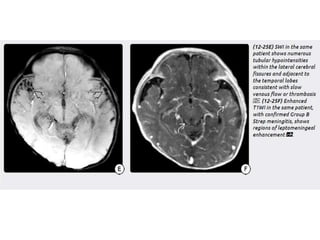
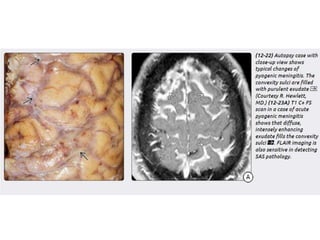
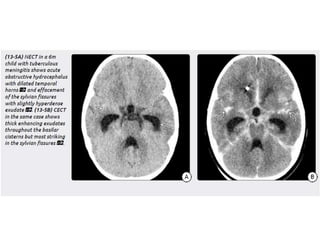
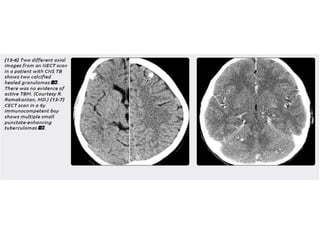
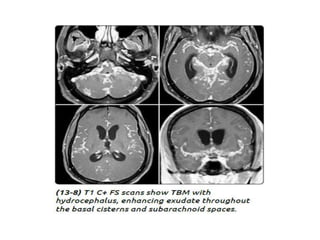
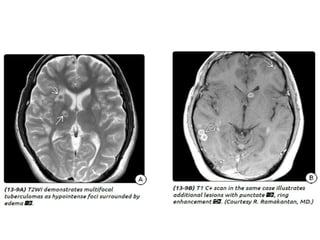
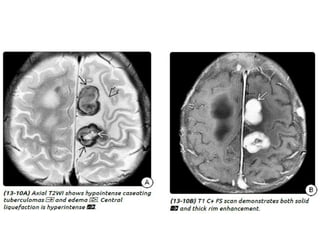
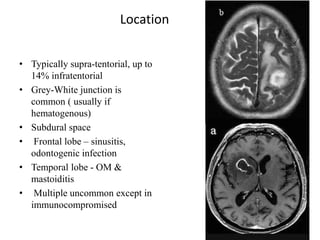
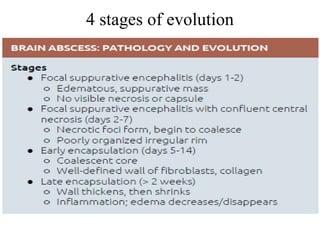
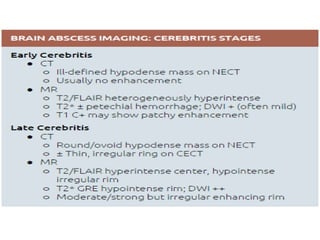
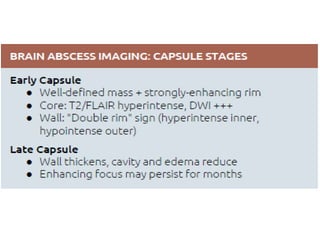
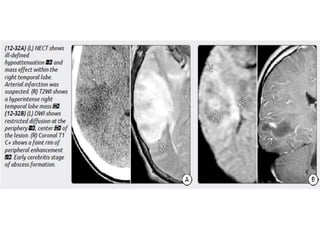
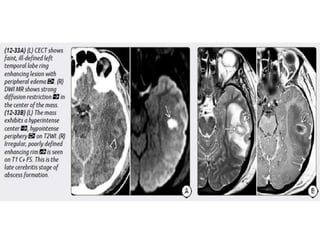
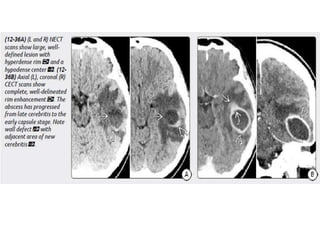
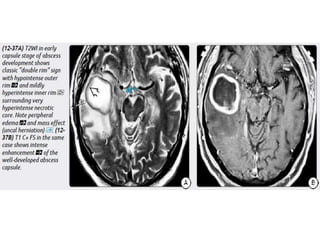
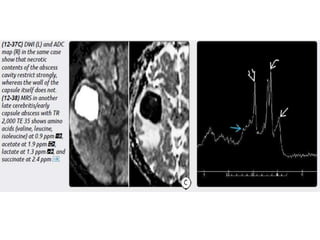
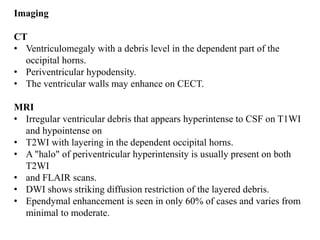
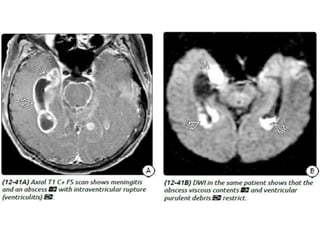
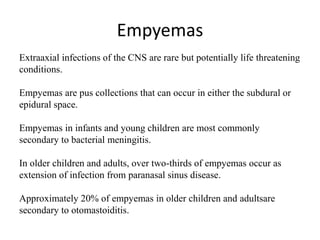
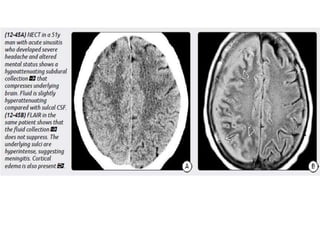
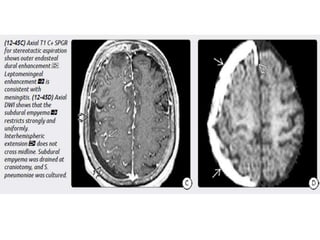
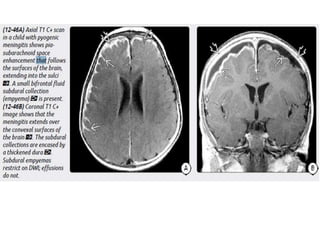
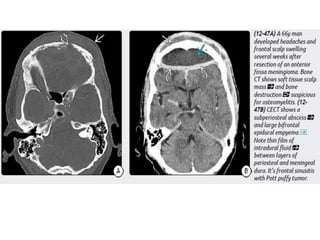
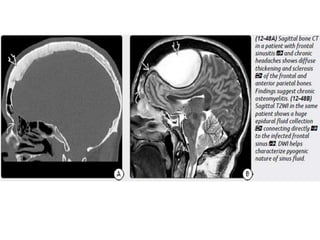
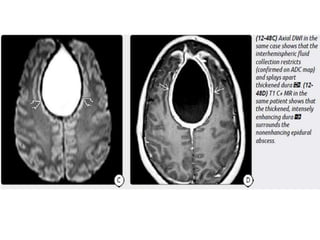
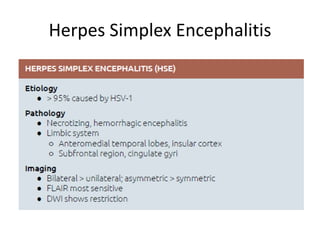
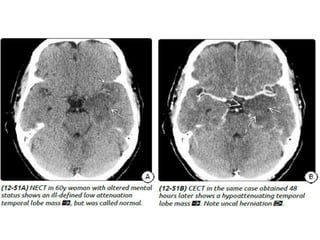
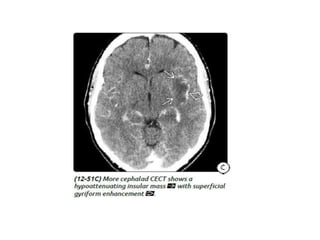
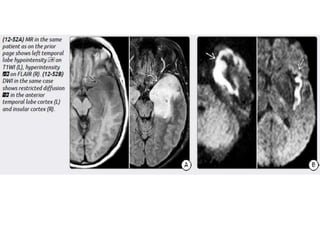
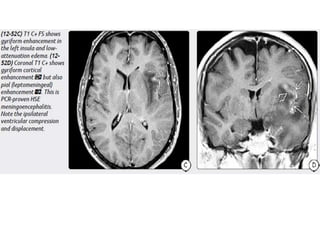
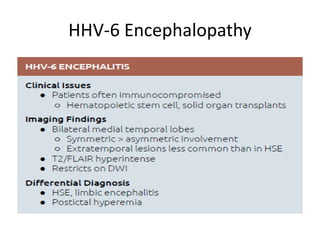
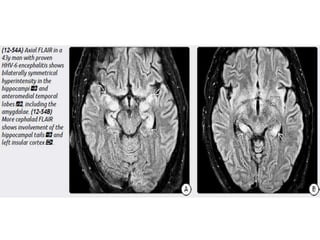
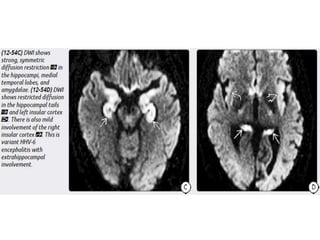
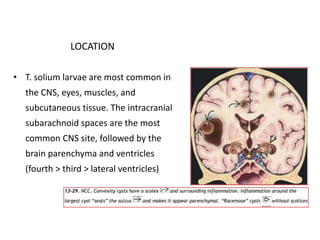
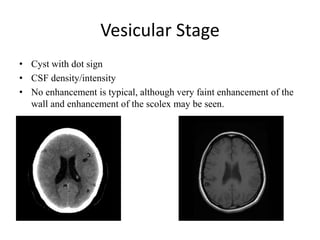
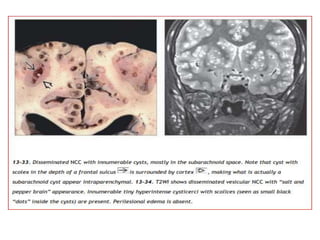
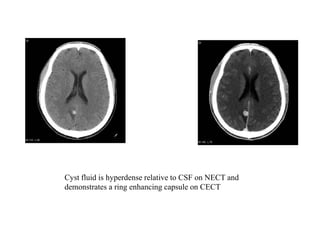
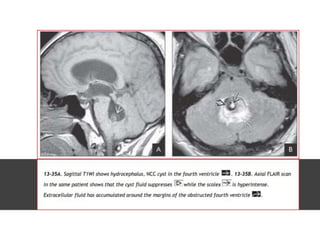
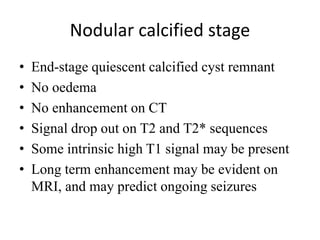
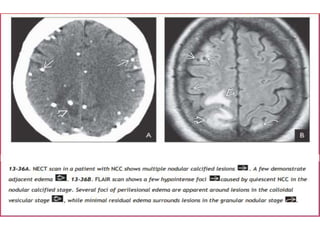
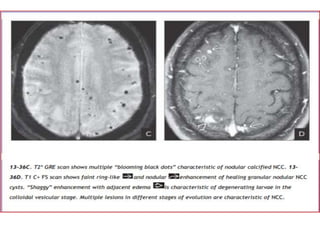
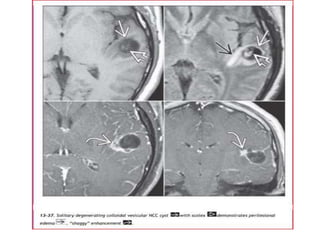
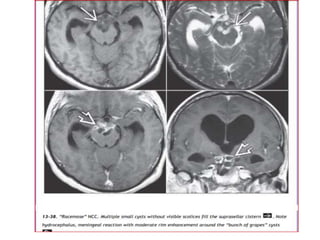
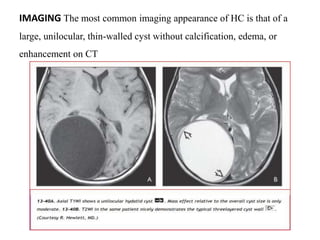
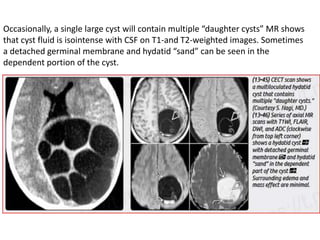
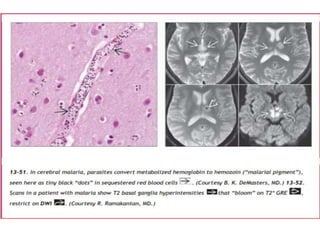
The document discusses various central nervous system infections, how they can be classified, their routes of entry and imaging appearances. It covers congenital infections including TORCH infections, acquired pyogenic infections such as meningitis, abscesses and ventriculitis. It also discusses viral, parasitic and fungal infections of the CNS. For each type of infection, the causative pathogens, locations, presentations and characteristic imaging findings are outlined.