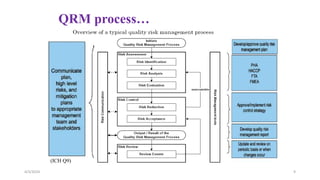
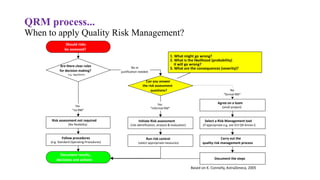
This document provides an overview of Quality Risk Management (QRM) in the pharmaceutical industry. It discusses the key principles and general process of QRM, including risk assessment, control, communication and review. The three main steps in the QRM process are risk identification, analysis and evaluation to assess risks, followed by risk control measures to reduce, accept or mitigate risks, and risk review to monitor risks over time. QRM aims to systematically minimize risks to product quality throughout a drug's lifecycle.