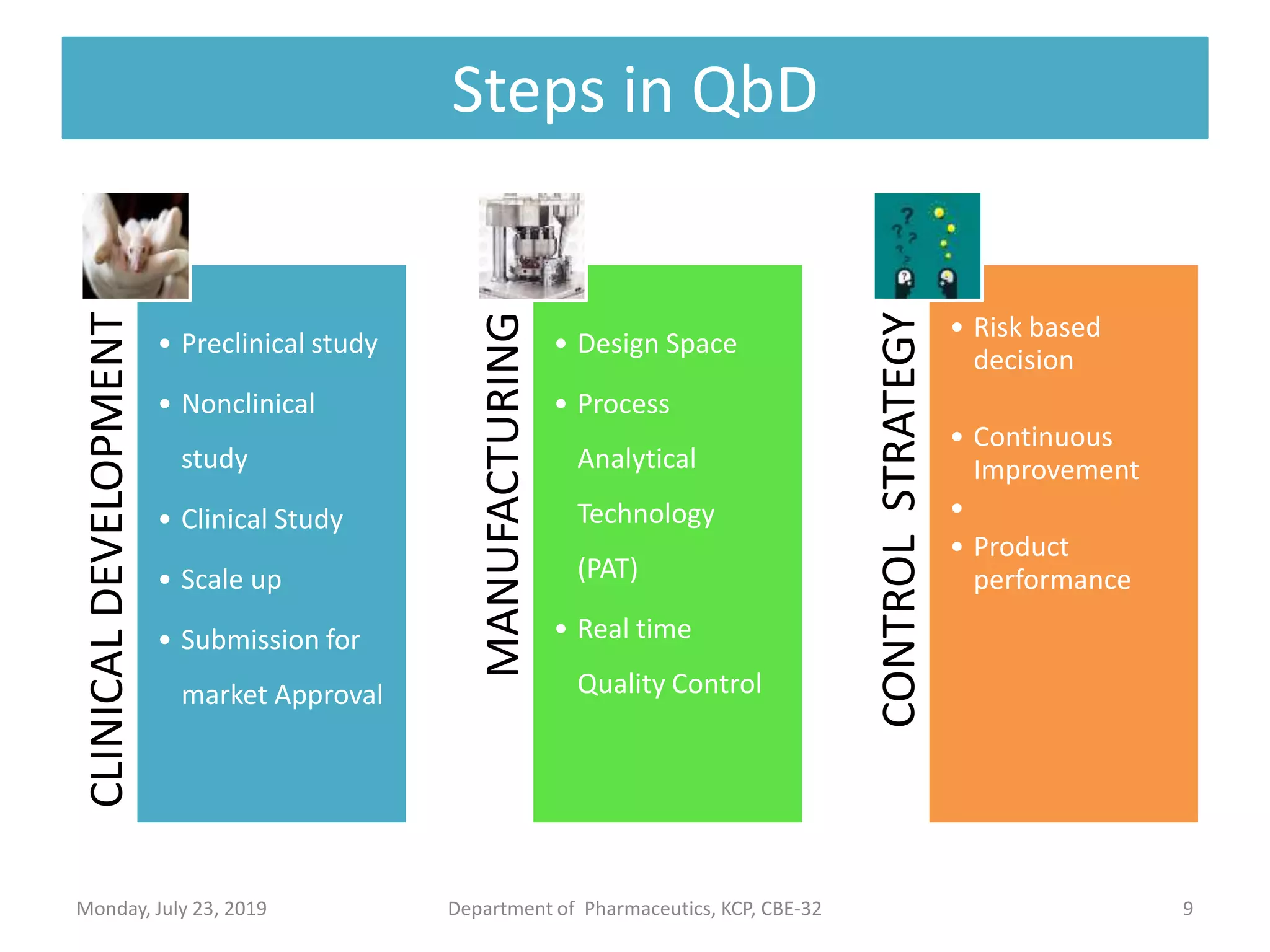
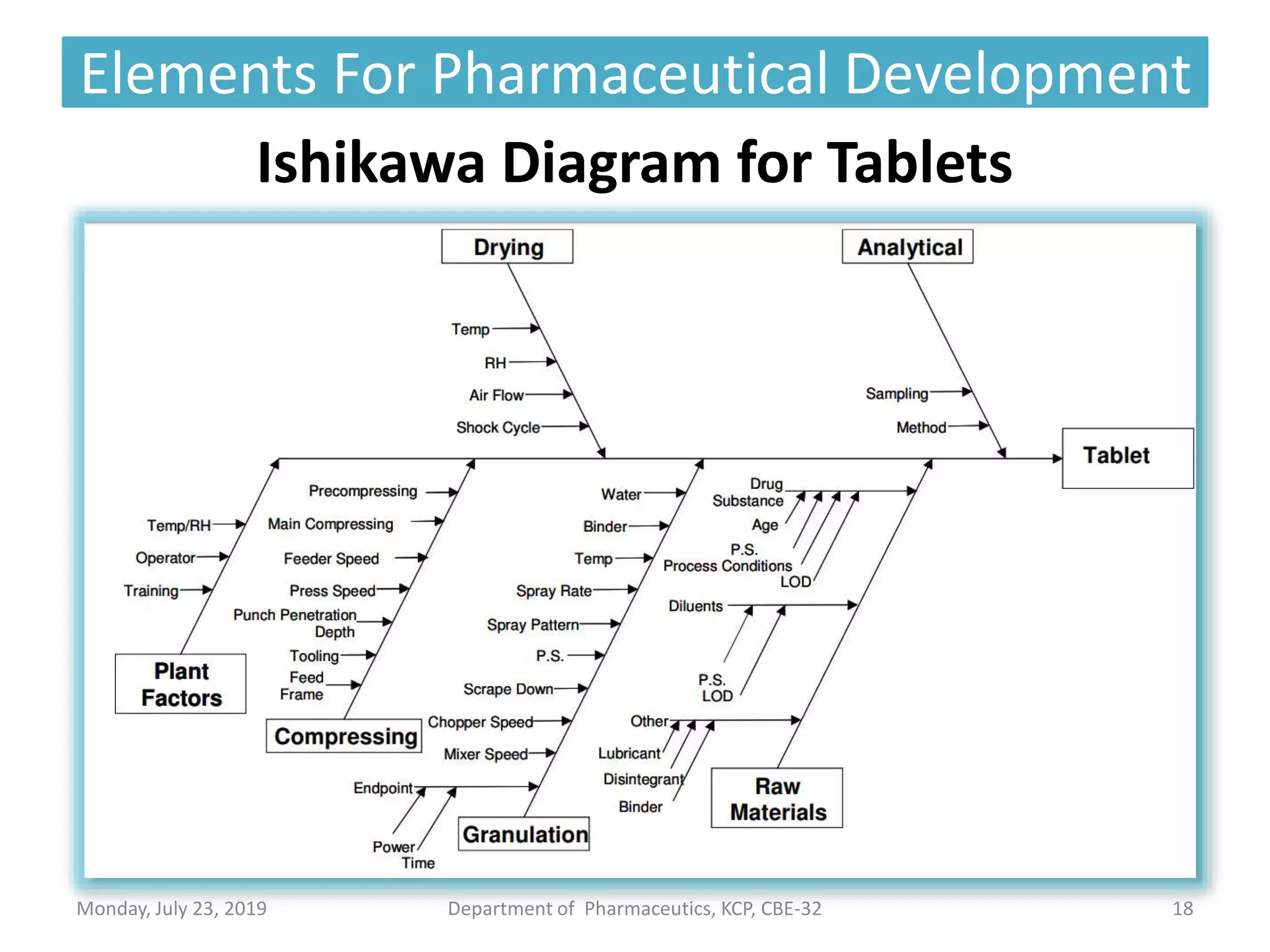
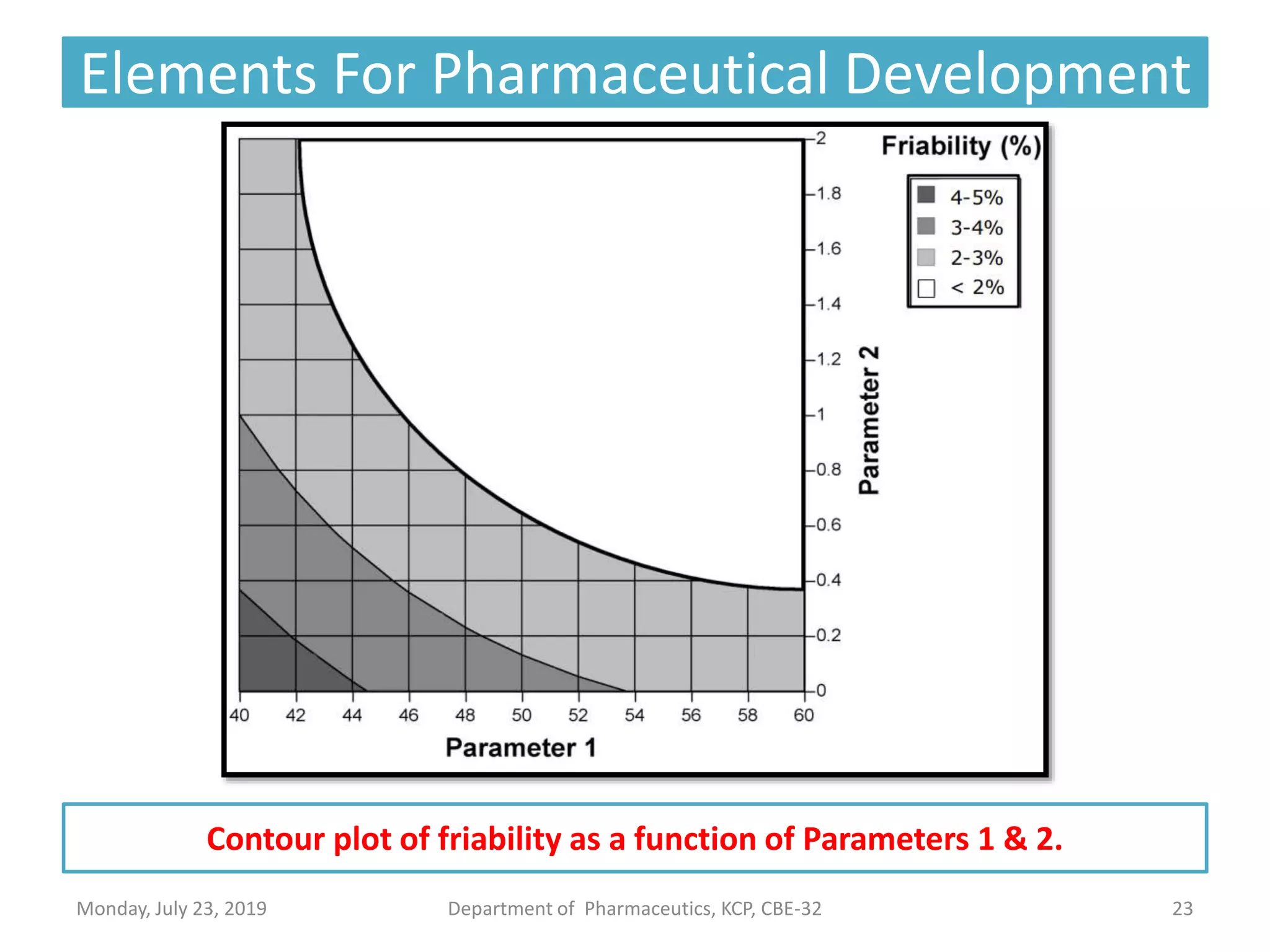
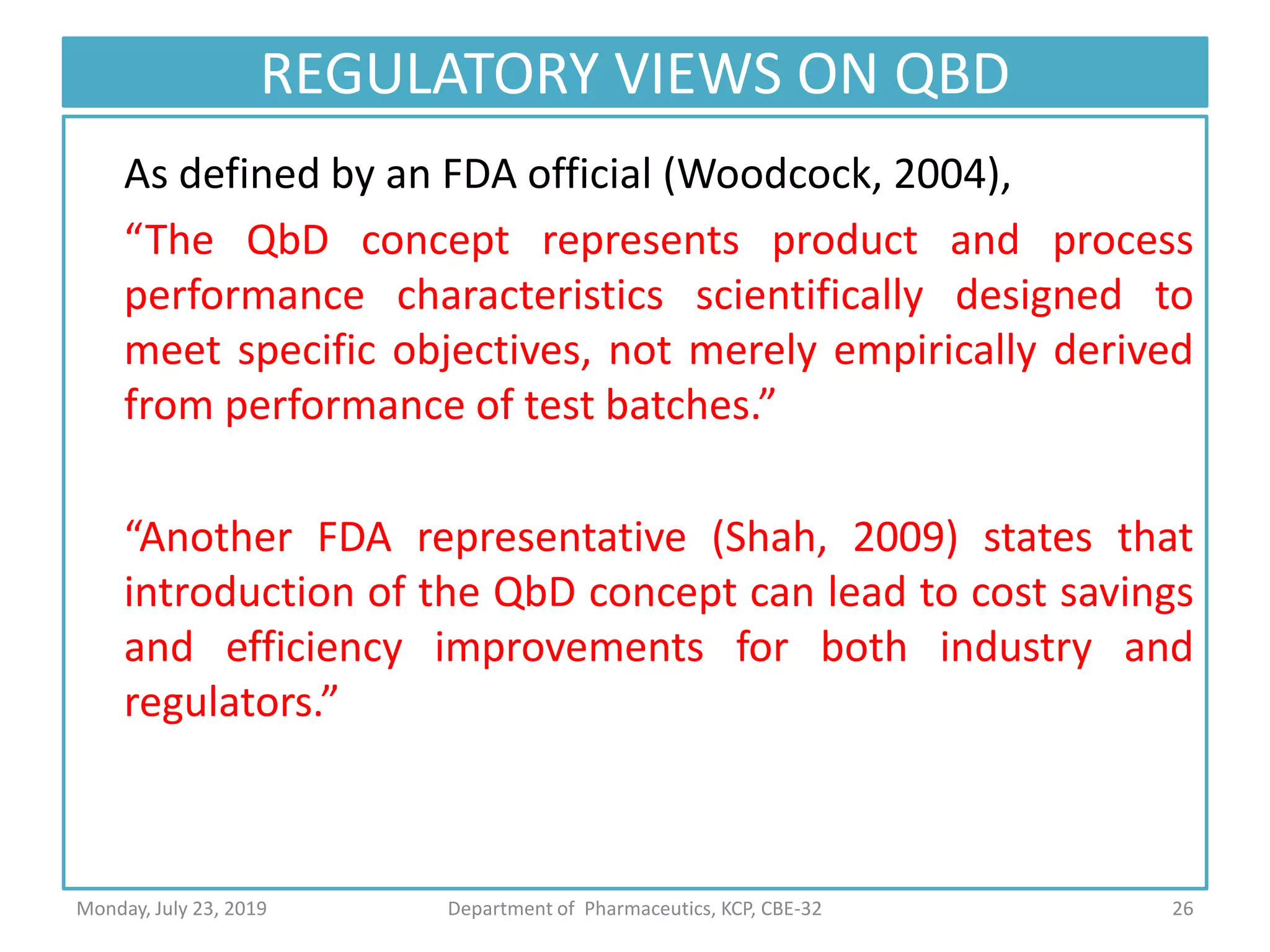
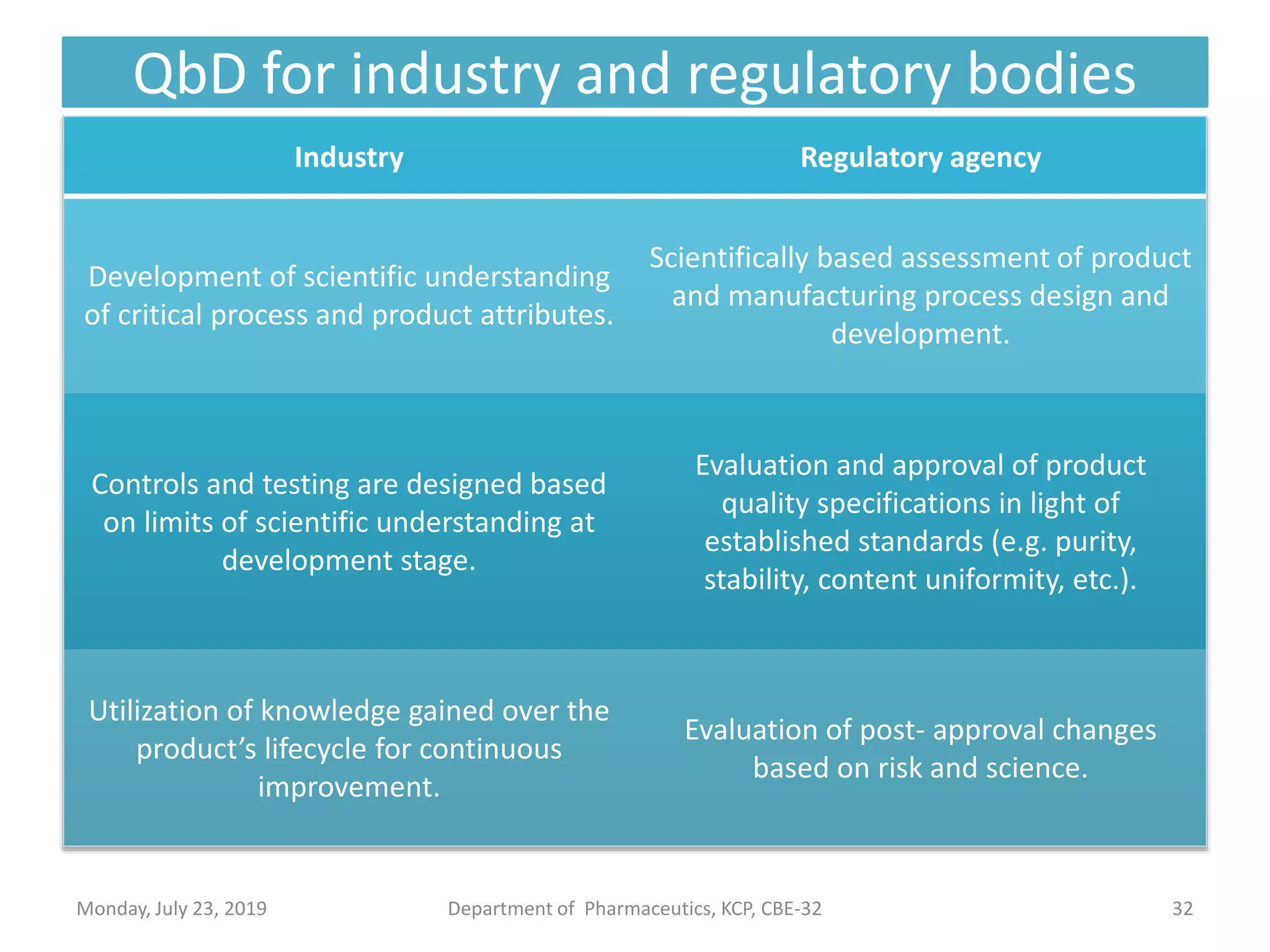
The document outlines the Quality by Design (QbD) approach in pharmaceutical development, emphasizing a systematic methodology that incorporates predefined objectives, product understanding, and risk management. It explains key concepts including the Quality Target Product Profile (QTPP), Critical Quality Attributes (CQA), and the importance of control strategies, risk assessments, and design space in enhancing product and process quality. Regulatory perspectives from FDA and EMA highlight the benefits of QbD for improving efficiency, reducing costs, and providing more focused regulatory processes.