The document discusses the Common Technical Document (CTD) and electronic CTD (eCTD) formats used for submitting registration documents to international regulatory agencies. The CTD format organizes documents into 5 modules: Module 1 contains administrative information specific to each region; Module 2 contains summaries of quality, non-clinical, and clinical information; Module 3 contains quality/manufacturing data; Module 4 contains non-clinical study reports; and Module 5 contains clinical study reports. The eCTD format is the electronic version of CTD, with documents in PDF format linked together via an XML backbone for easier navigation and review compared to the paper CTD format.

![CTD
• Common Technical Document [CTD]: It is an
format set by ICH which was agreed by the
Regulatory Agencies of Europe , Japan & the U.S.
• The FDA characterized the CTD as “An
information package of clinical, non clinical ,
manufacturing , technical data in the same
content that would be submitted for registering
new drugs in all 3 ICH regions i.e. U.S,European
Union and Japan](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-2-2048.jpg)
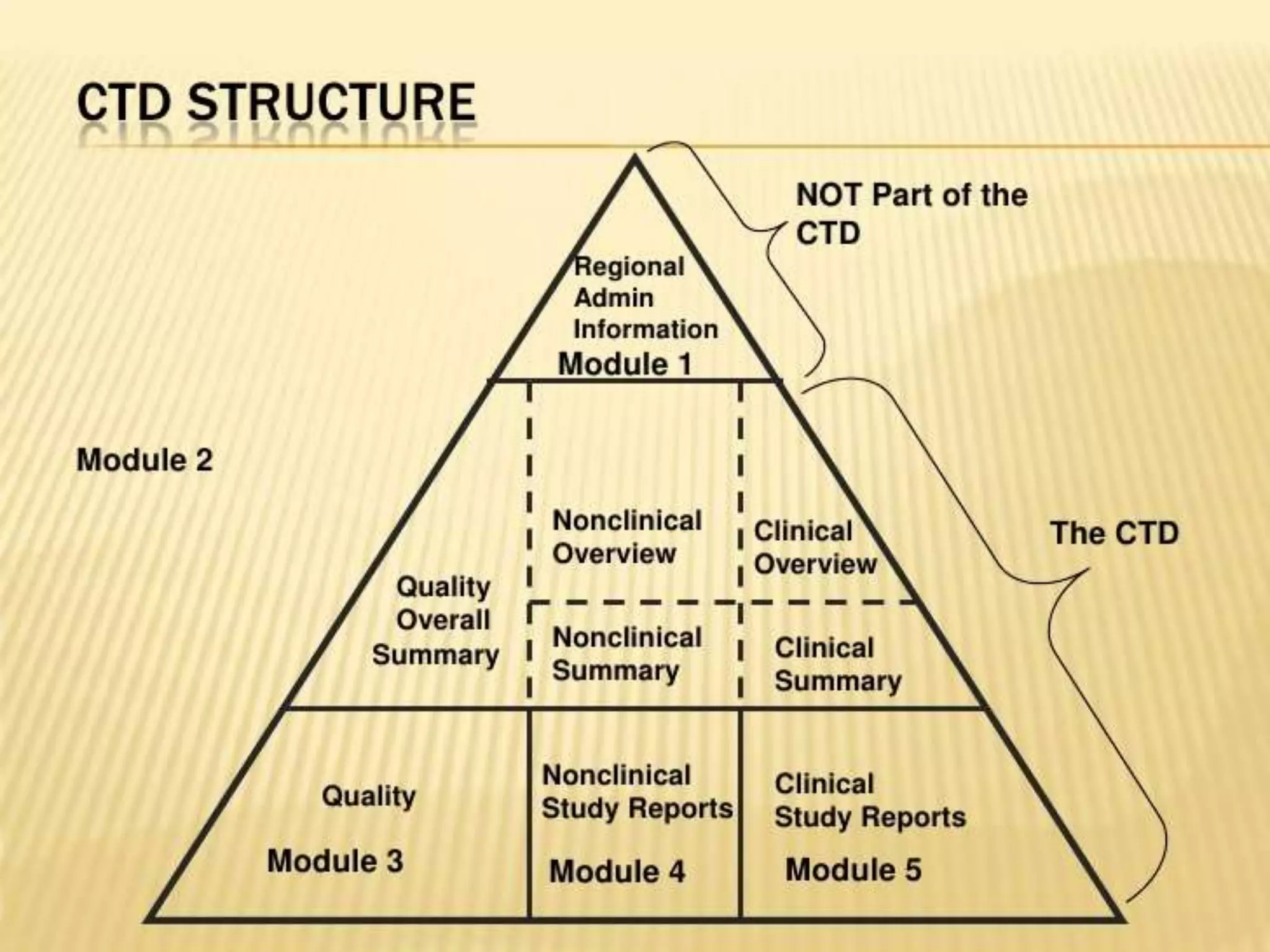
![ORGANISATIONS OF CTD:
• It should be organized into 5 modules
- Module 1 – Administrative Information
[Region Specific]
-Module 2 – CTD Summaries[QOS]
-Module 3 – Quality[CMC]
-Module 4 – Non clinical study reports
-Module 5 – Clinical study reports](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-4-2048.jpg)
![Module 1
Administrative Information [Region
specific]
• This module should contain documents
specific to each region
• Ex : Application form regarding the
prescribing information, proposed label
• This module is not part of the CTD.
• The content & format of this module can be
specified by the relevant regulatory
authorities.](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-5-2048.jpg)
![Module 2
CTD Summaries [QOS]
• It should begin with a general introduction to
the pharmaceutical , including its
pharmacological class , mode of action &
proposed clinical use. i.e. information should
not exceed one page
• It contain 7 sections in the following order:
- 2.1 CTD TOC [Module 2 – 5] [Table Of
Content]
- 2.2 CTD Introduction
- 2.3 Quality Overall Summary](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-6-2048.jpg)
![- 2.4 Nonclinical overview
- 2.5 Clinical overview
- 2.6 Non clinical summary
- 2.7 Clinical summary
• The organization of these summaries is
described in 3 separate documents:
A] M4 Q – The CTD quality
B] M4 S - The CTD Safety
C] M4 E - The CTD Efficacy](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-7-2048.jpg)
![Module 3
Quality [CMC]
• 3.1 TOC of Module 3
• 3.2 Body of Data
- 3.2.S -Drug substance
- 3.2.P – Drug product
- 3.2.A – Appendices
- 3.2.R – Regional information
• 3.3 Literature references](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-8-2048.jpg)
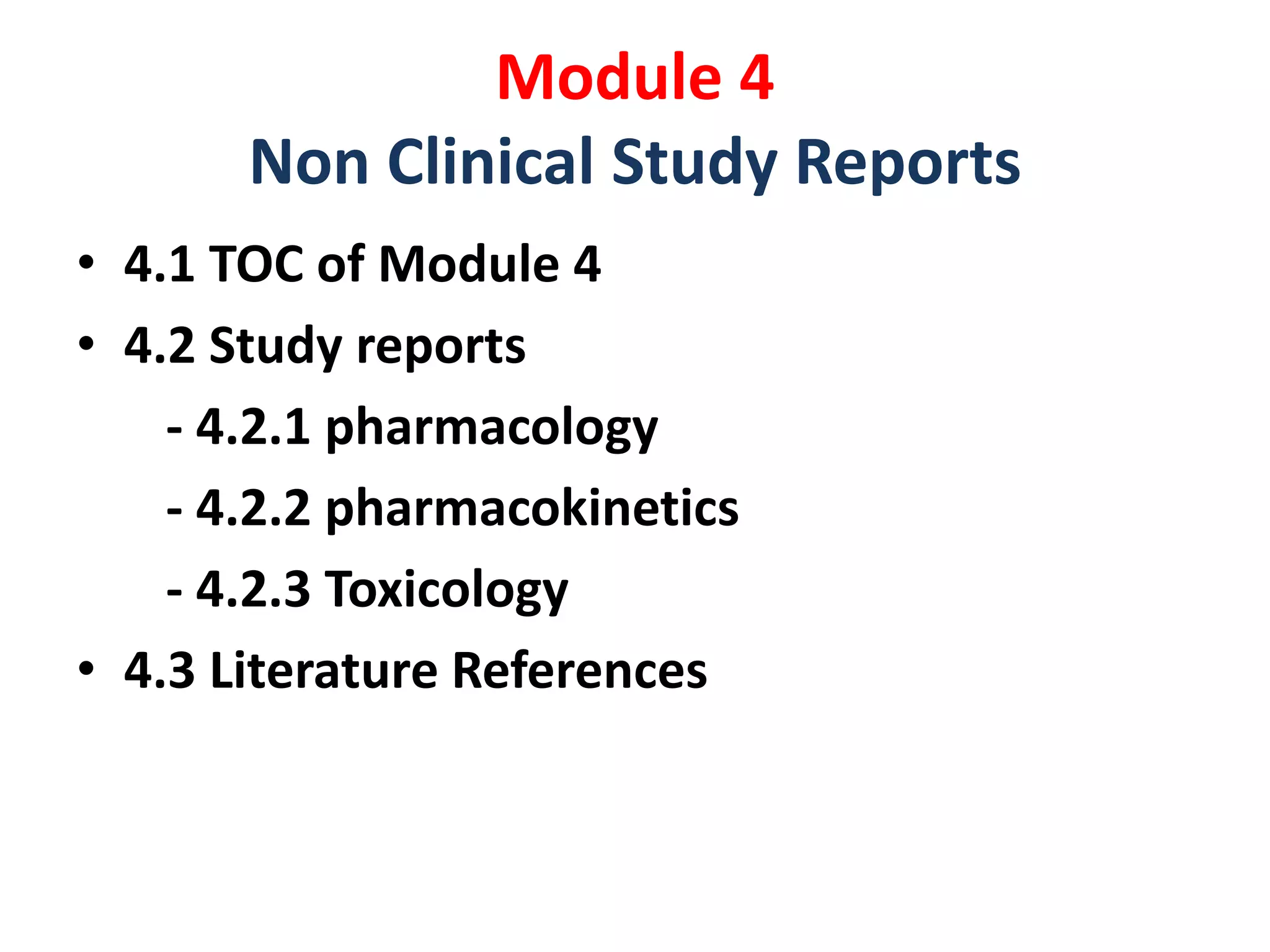
![• 5.1 TOC of Module 5
• 5.2 Tabular listing of clinical studies
• 5.3 Clinical study reports
-5.3.1 Repots of biopharmaceutical study[BA-BE]
-5.3.2 Reports of PK [biomaterial] study
-5.3.3 Reports of PK studies
-5.3.4 Reports of PD studies
-5.3.5 Reports of Efficacy and safety studies
-5.3.6 Reports of Post marketing experience
-5.3.7 Case Report forms & Individual patient listings
• 5.4 Literature References
Module 5
Clinical Study Reports](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-10-2048.jpg)
![e CTD
• It is electronic version of CTD , so called as
electronic common technical document
[e CTD]
• e CTD composed of 2 types of specification
- Content specification – As defined by ICH
- Technical specification- Electronic softwares
CTD TOC [pdf] [paper]
e CTD XML Backbone](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-11-2048.jpg)

![e CTD Characteristics
• Structure
-All Modules 1 to 5 have granularity options[
level of detail a document has ]
-PDF documents linked via XML backbone
-Increased document granularity.
-Transparency of entire submission
-Ease of navigation and review](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-13-2048.jpg)
![COMPARING PAPER CTD AND e CTD
Paper CTD e CTD
Compiled electronically with volumes ,
tabs , slipsheets then printed to paper
Compiled electronically with e documents
in folders
Paper volumes must be A4 e Documents can be A4 or US letter size
CTD navigation by TOC s and volume e CTD navigation by XML backbone
Cross references includes target CTD
section number
Cross references are hyperlinked to
targets
Manual document navigation by TOC s,
page numbers, and caption cross
references
Electronic document navigation by TOC s
,bookmarks and hyperlinks
Submitted in binders in boxes on pallets
by trucks
Submitted on CD[ or DVD] or by email or
portal](https://image.slidesharecdn.com/ctdandectd-180226180436/75/Ctd-and-e-ctd-15-2048.jpg)
