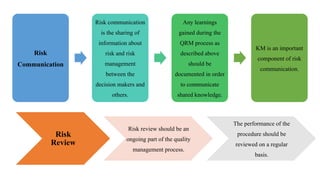
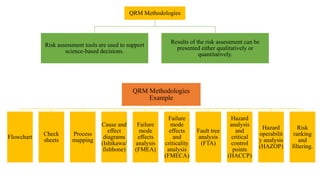
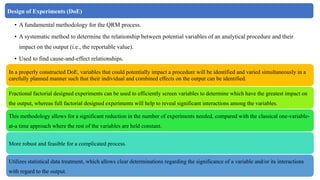
The document outlines the application of a Quality Risk Management (QRM) process to analytical control strategies, focusing on identifying, analyzing, evaluating, and controlling risks associated with the quality of reportable values in analytical procedures. It details the iterative nature of risk management, emphasizes the importance of mitigating risks to acceptable levels, and discusses various methodologies and tools to support risk assessment and decision-making. The goal is to maintain the quality and reliability of analytical procedures through systematic risk communication and ongoing review.