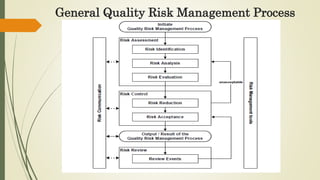
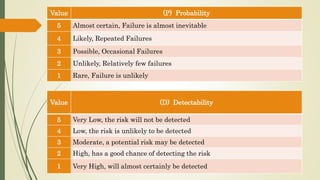
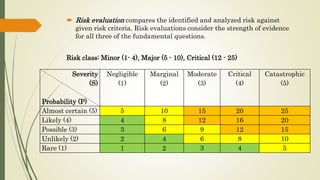
Quality Risk Management (QRM) is a systematic process for managing risks to product quality throughout its lifecycle, aiming to ensure patient safety and product efficacy. It involves risk assessment, control, communication, and review, with interdisciplinary teams analyzing potential hazards and implementing appropriate measures. The methodology supports a scientific approach, utilizing various tools to evaluate and mitigate risks related to product development and manufacturing.