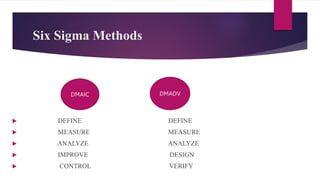
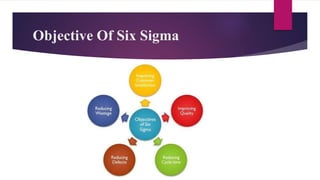
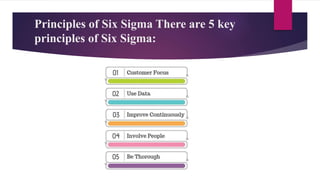
This document provides an overview of quality management systems and Six Sigma. It discusses basics of quality management including definitions of quality, dimensions of quality, and the scope of pharmaceutical quality management systems. It then introduces Total Quality Management (TQM) and its principles of continuous improvement. Six Sigma is defined as a statistical approach to process improvement, and its DMAIC methodology is explained. The key principles of Six Sigma are outlined as focusing on customer requirements, using data to identify process variation, improving processes to eliminate variation, involving multidisciplinary teams, and maintaining flexibility.