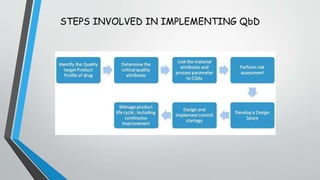
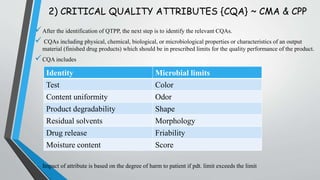
This document discusses Quality by Design (QbD), a systematic approach to pharmaceutical development that emphasizes product and process understanding based on sound science and quality risk management. It outlines the key elements of QbD including quality target product profiles, critical quality attributes, critical material attributes, critical process parameters, design space, control strategy, and product lifecycle management. Risk assessment tools and process analytical technology are also described as important tools that can be utilized in a QbD approach.