Embed presentation
Downloaded 110 times





















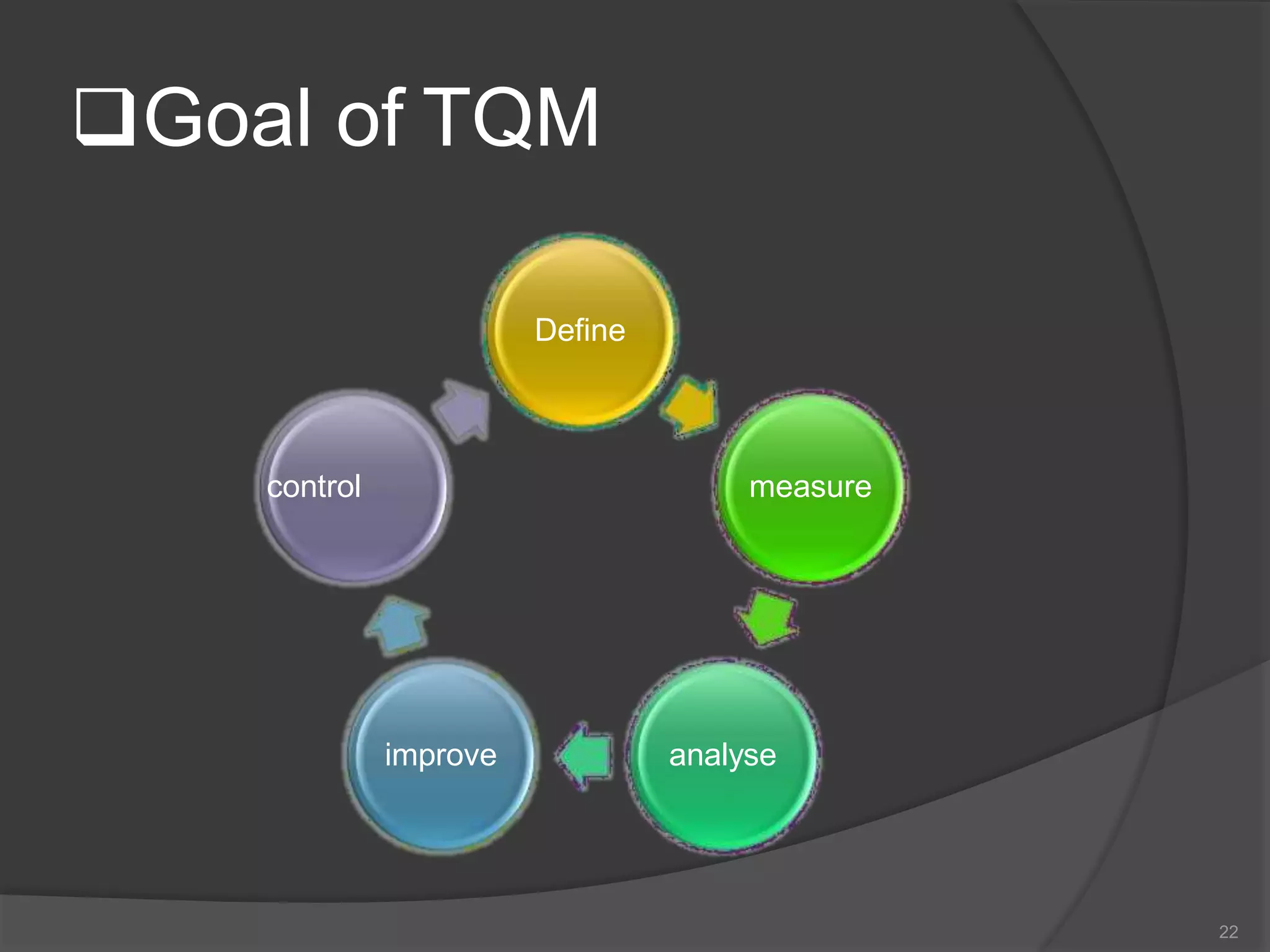
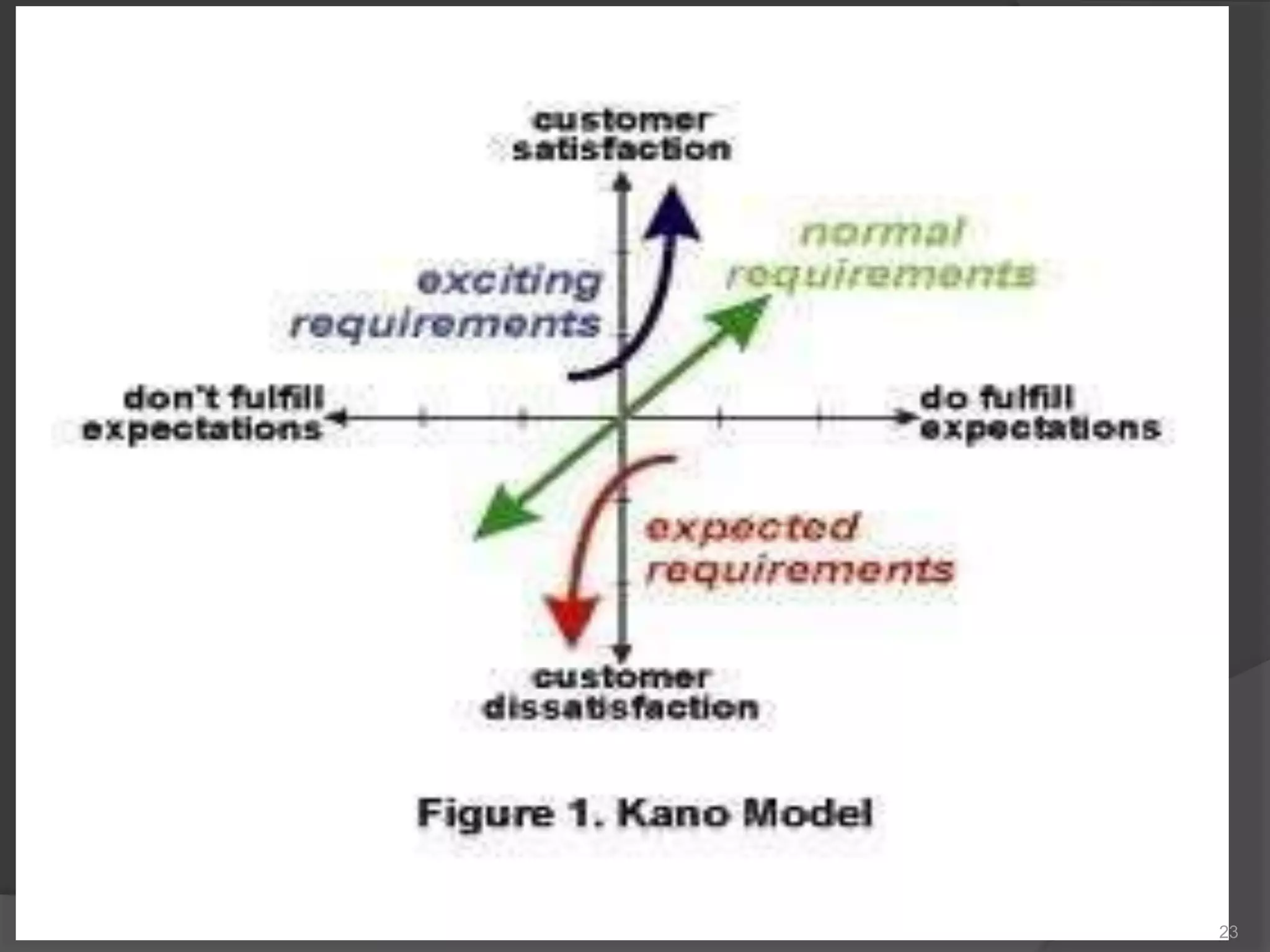
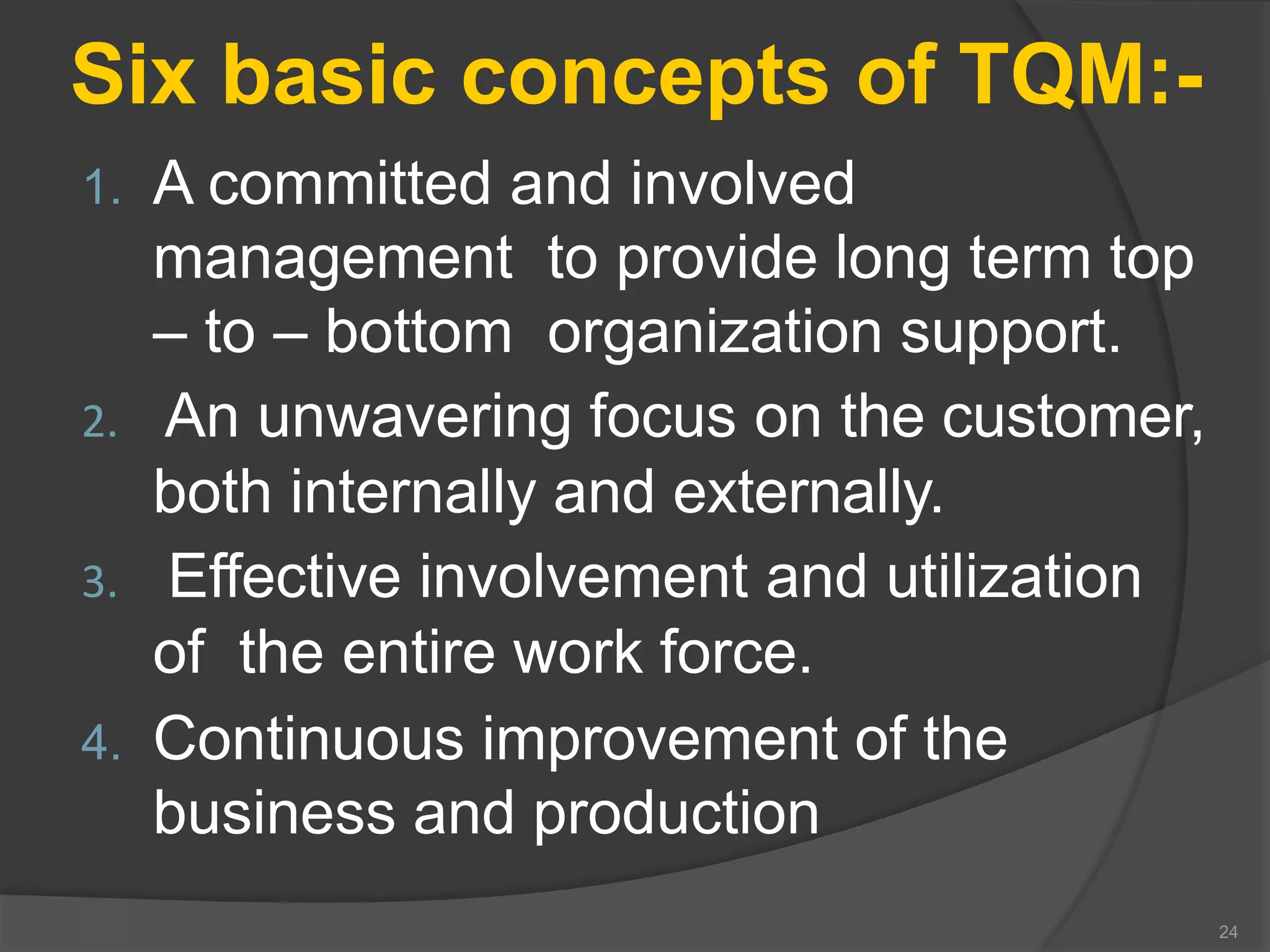
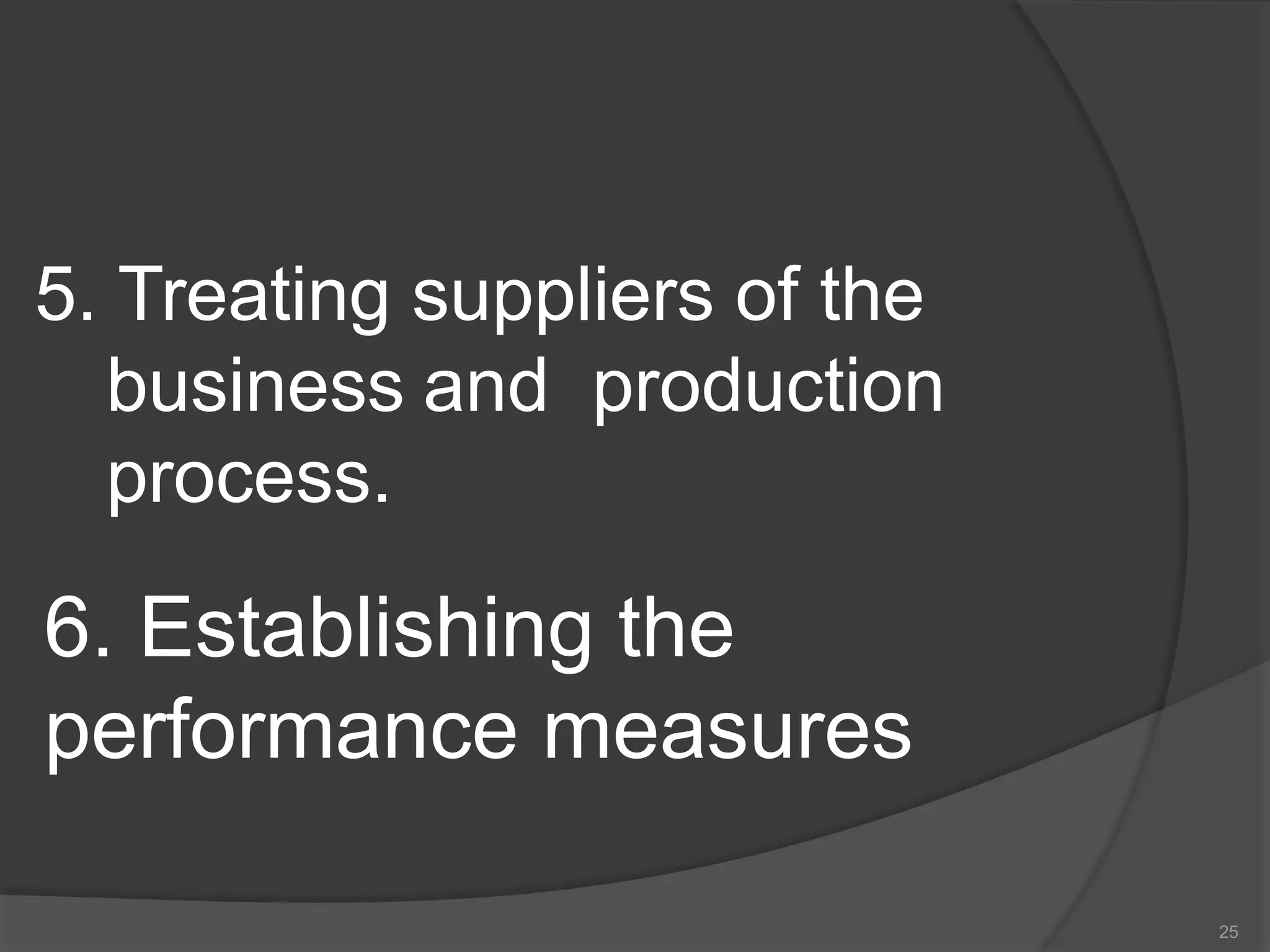
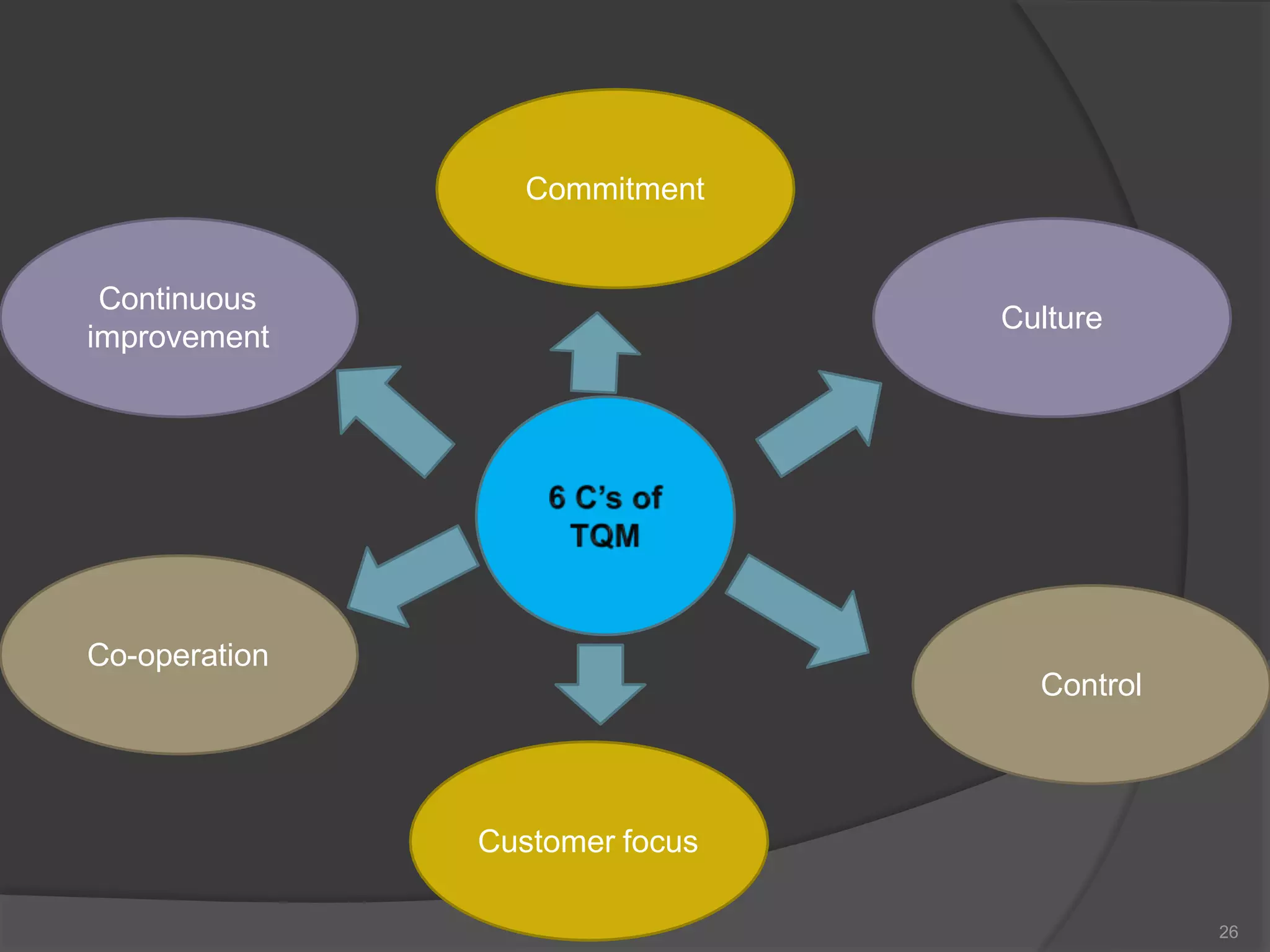















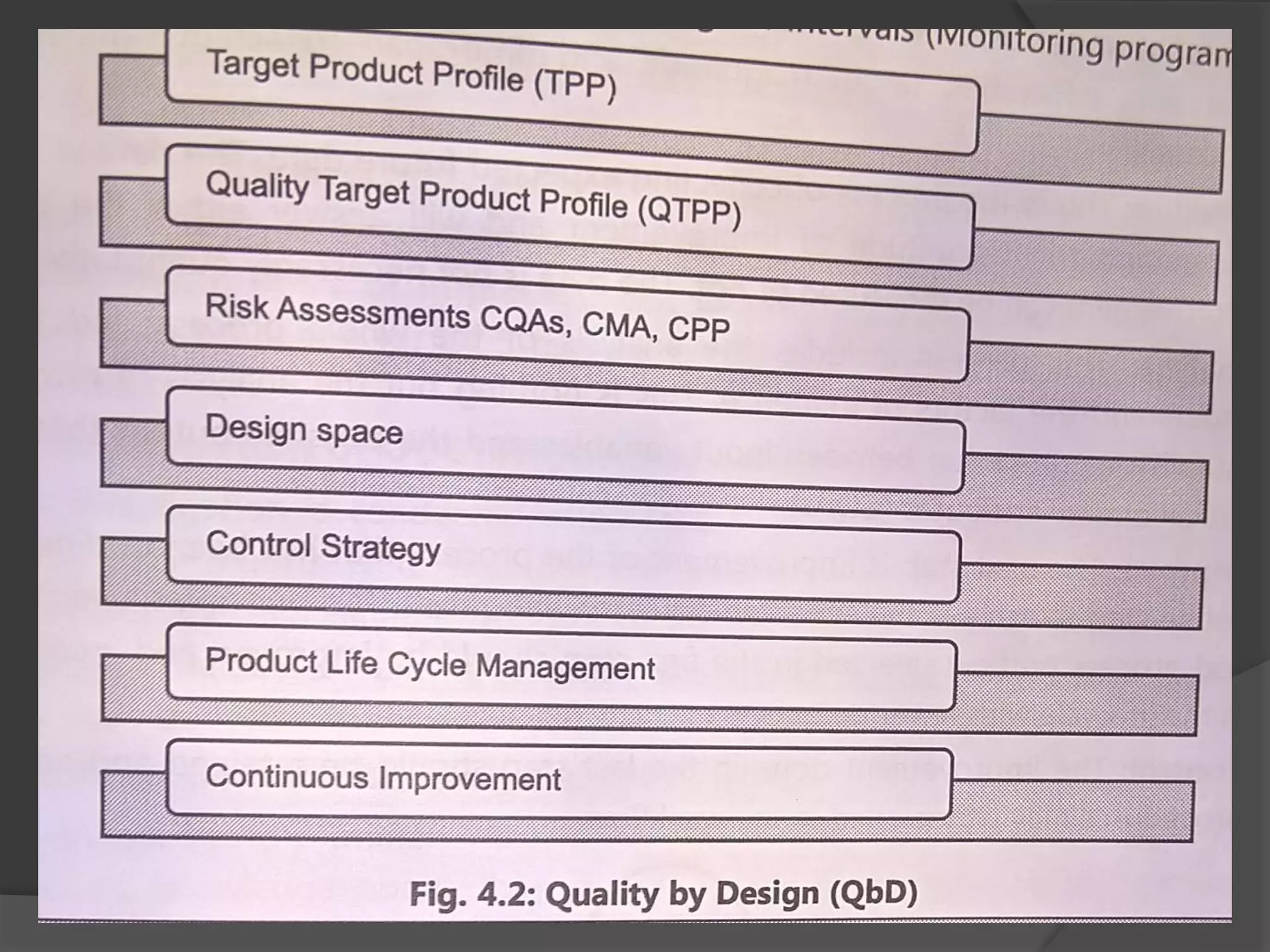




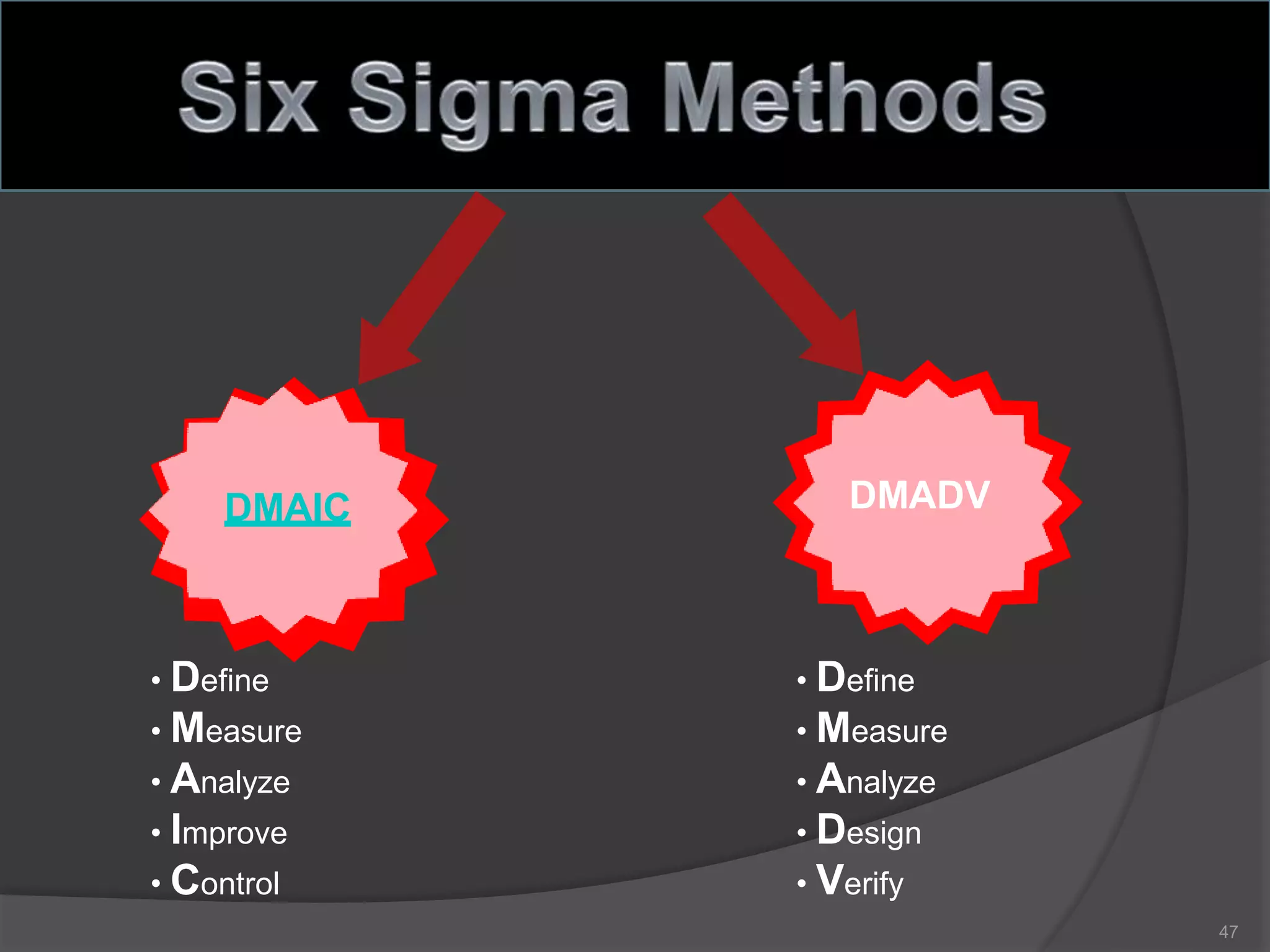
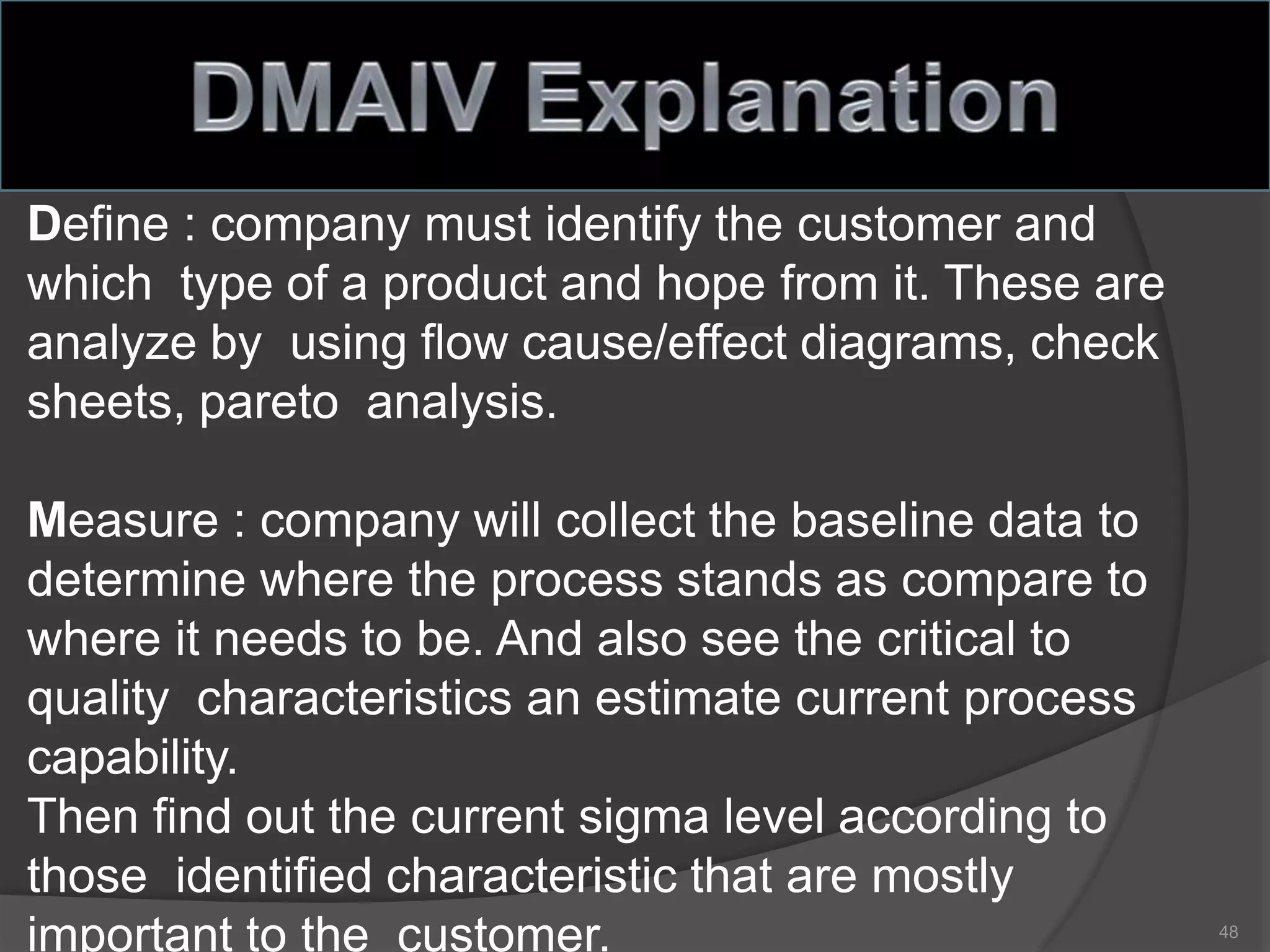





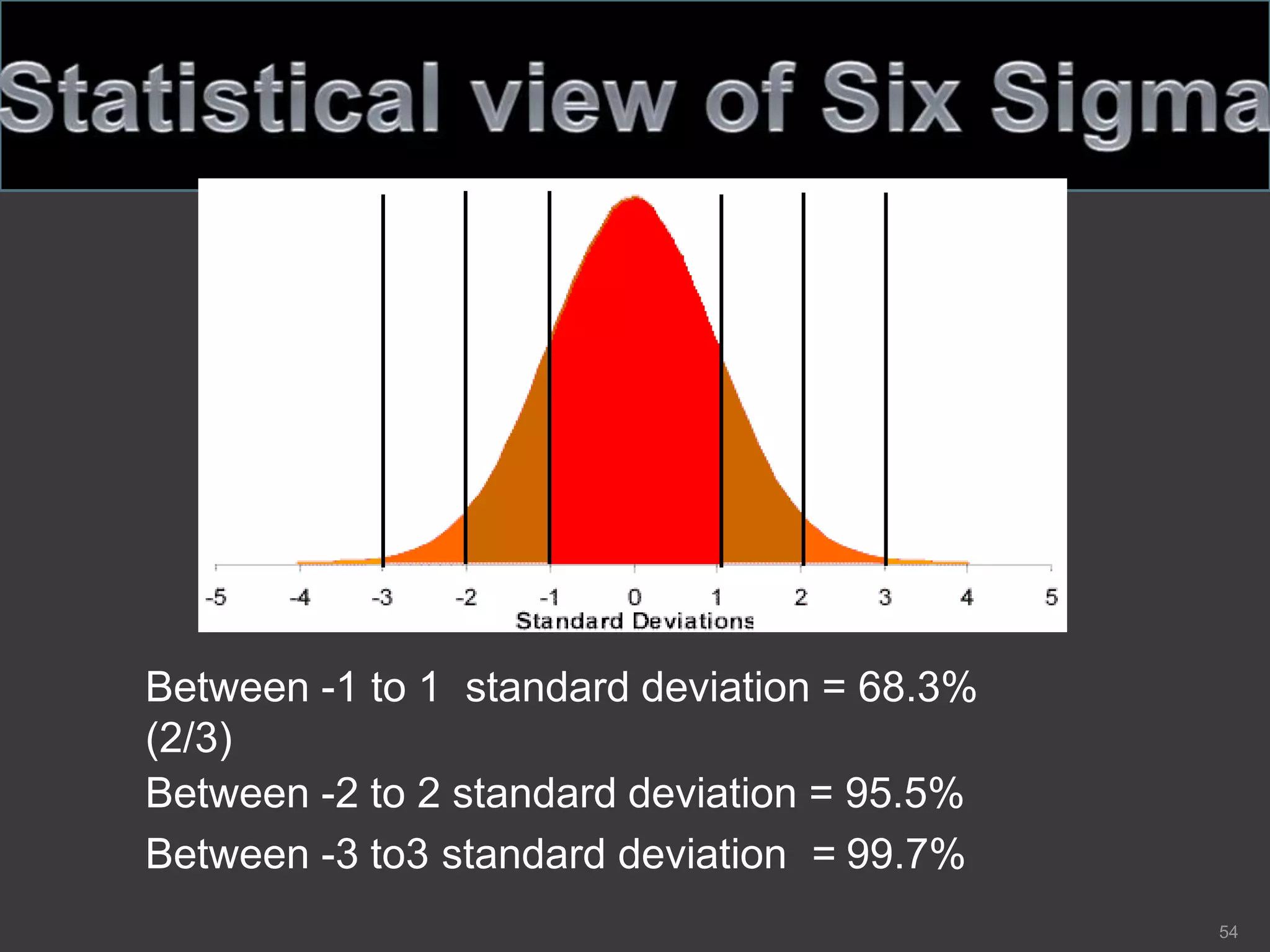









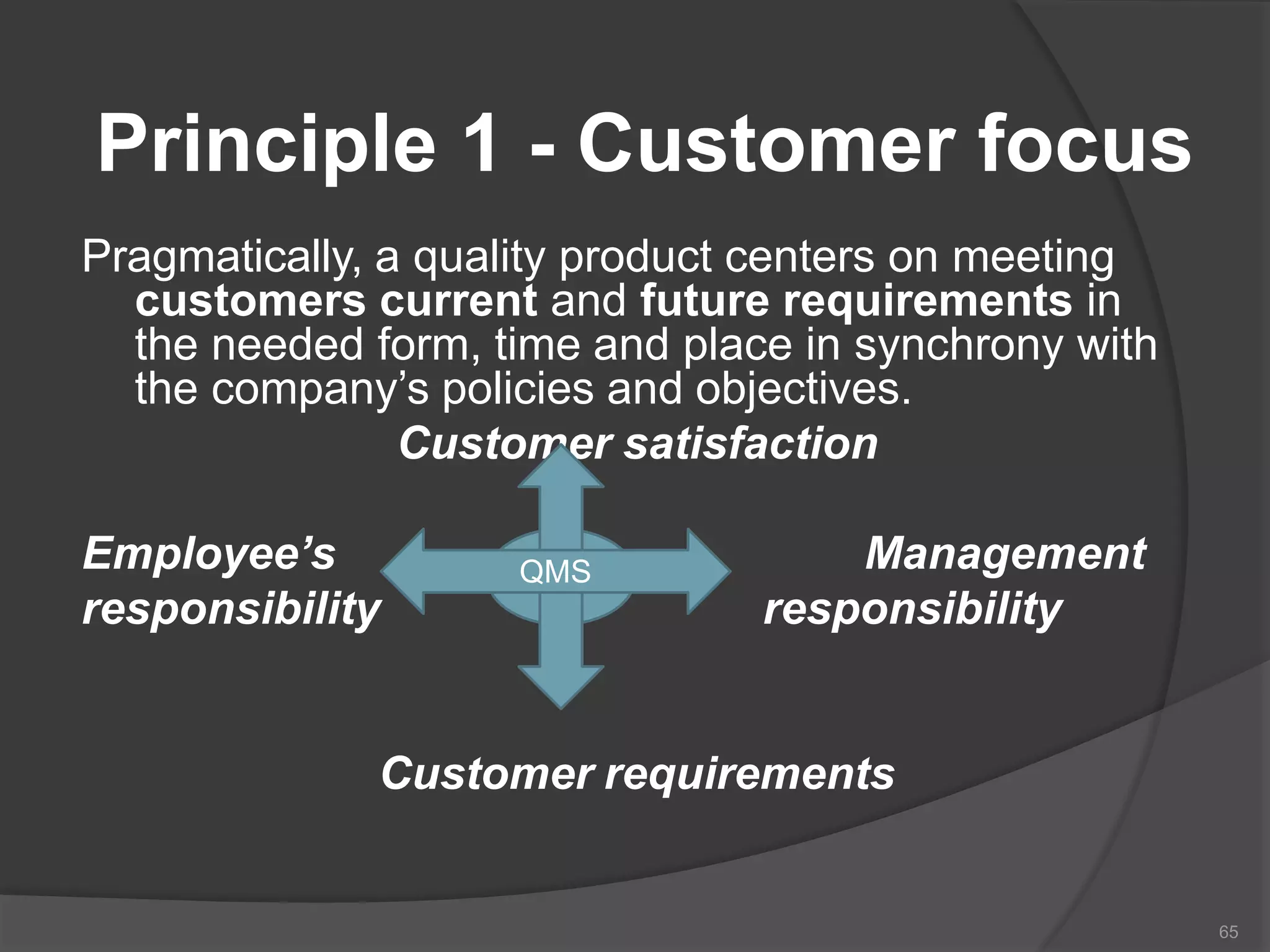

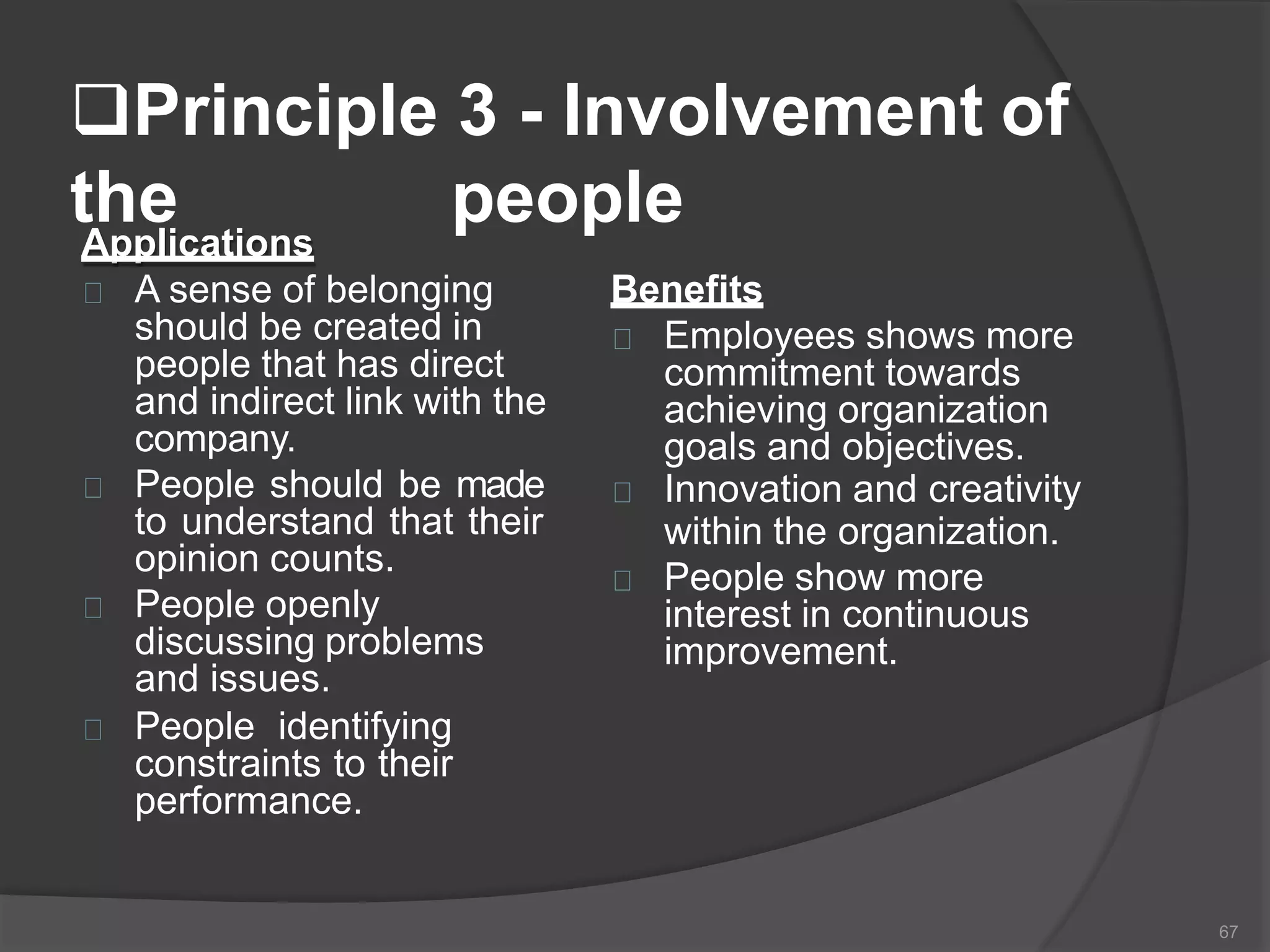

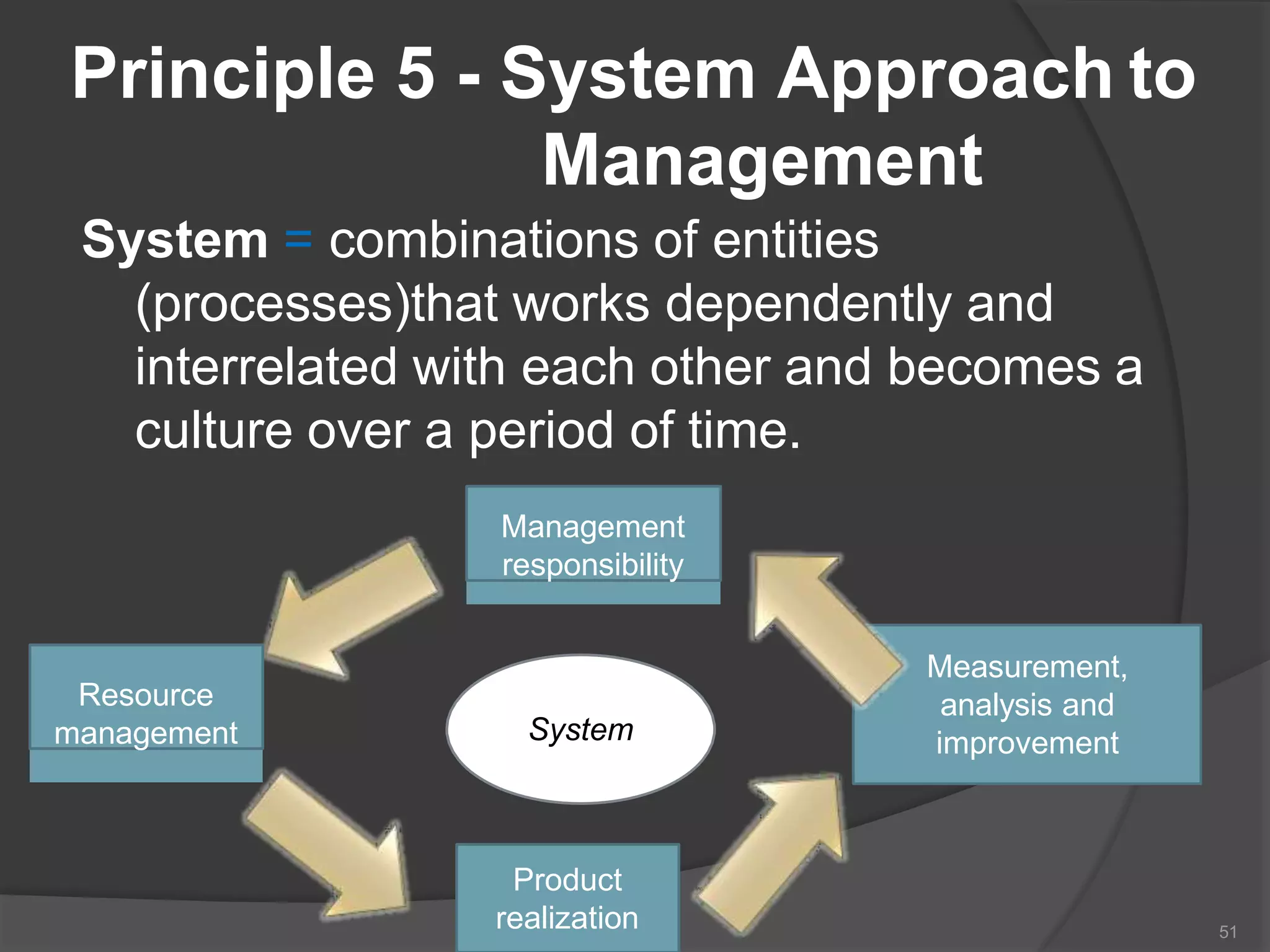

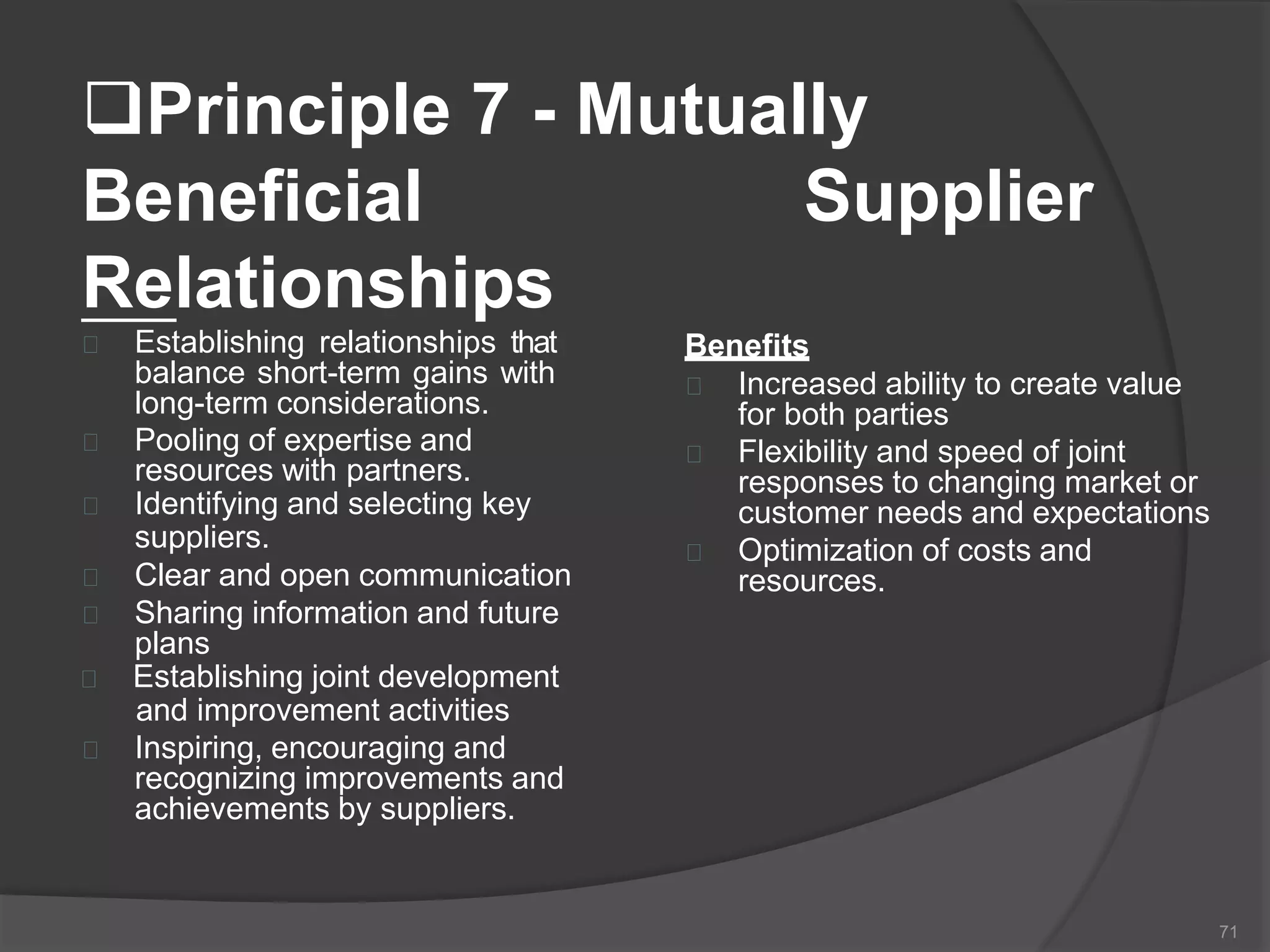































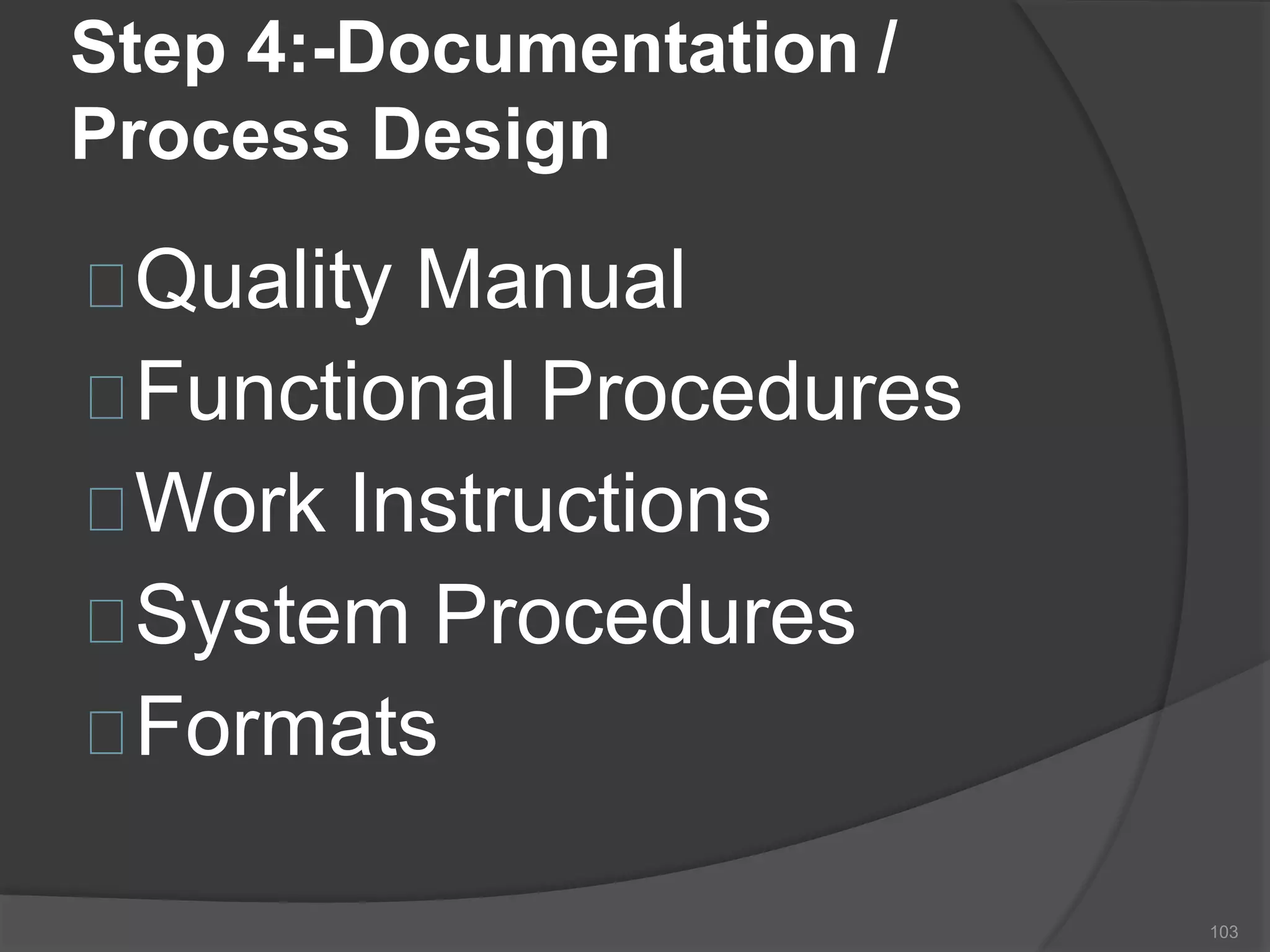







































The document outlines the fundamentals of a Quality Management System (QMS) in the pharmaceutical industry, emphasizing the importance of maintaining product quality and safety through adherence to regulations and guidelines such as those from the FDA and ICH. It discusses the roles of Quality Assurance (QA) and Quality Control (QC), the scope of pharmaceutical QMS, and introduces concepts such as Total Quality Management (TQM) and Quality by Design (QbD). Additionally, it covers the significance of methodologies like Six Sigma and the International Organization for Standardization (ISO) standards in ensuring quality and continuous improvement in pharmaceutical processes.













































































































































