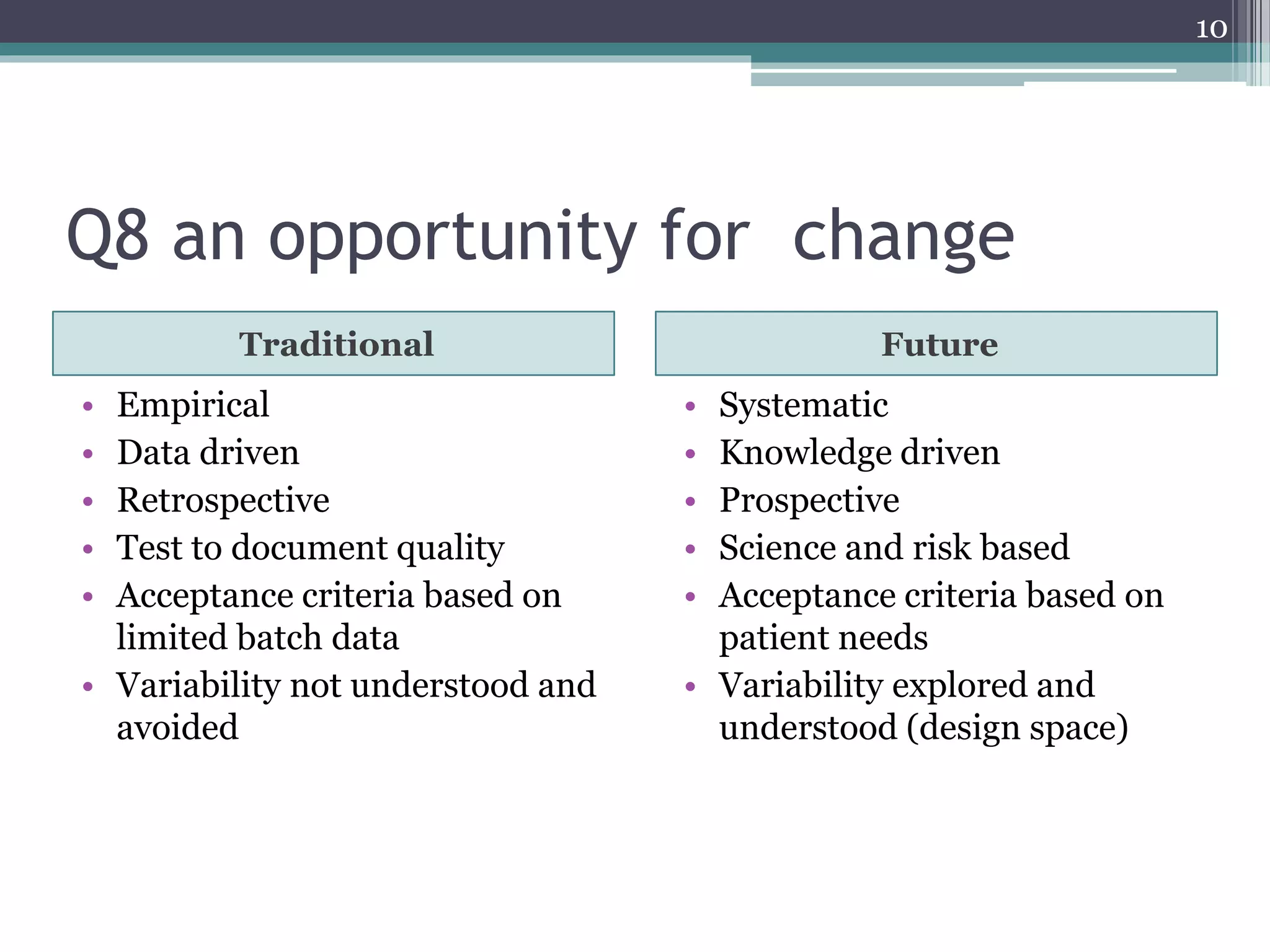
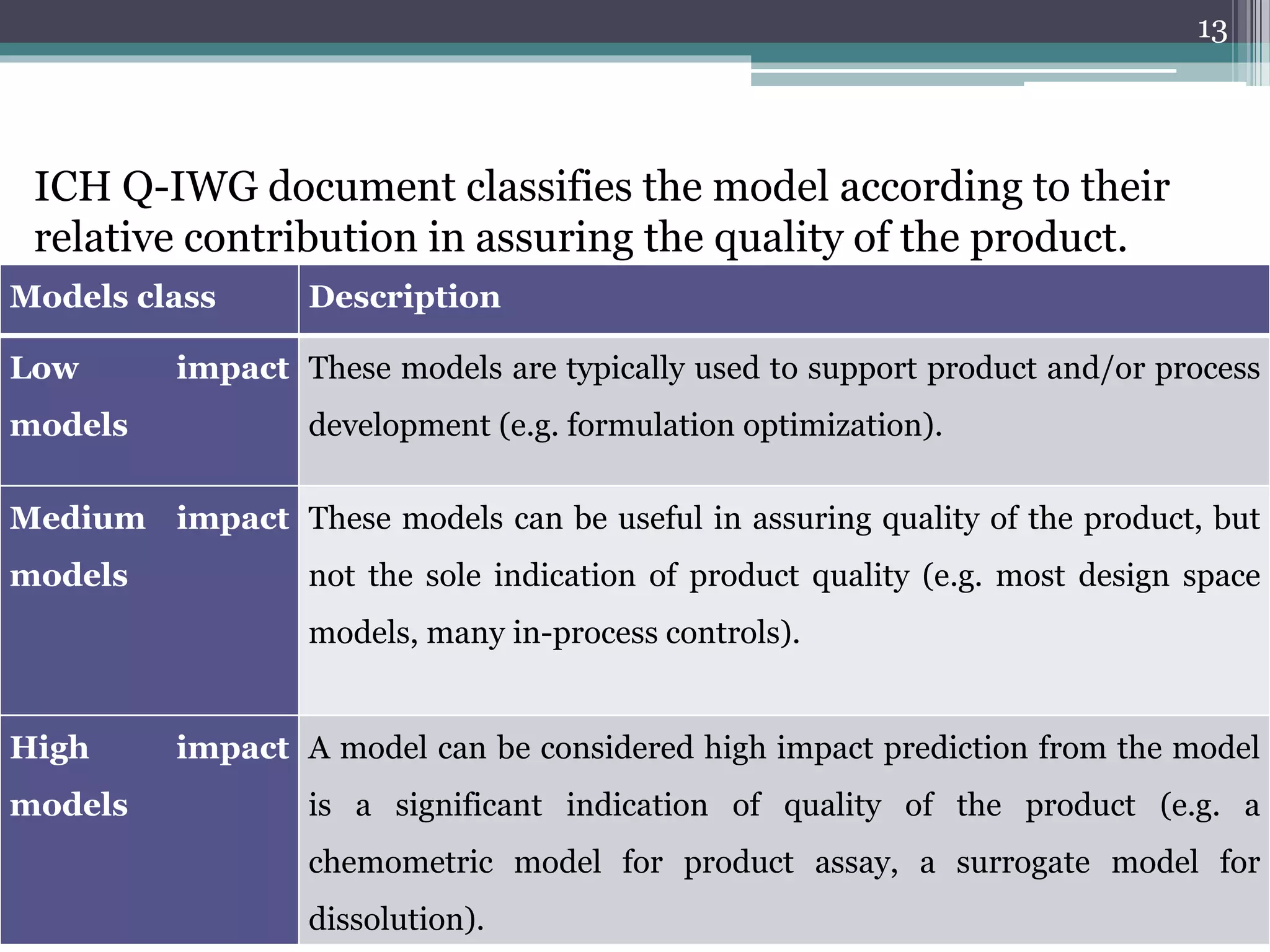
The document provides an overview of ICH Q8 guidelines for quality by design (QbD) in pharmaceutical development. It discusses key aspects of the QbD framework including defining critical quality attributes and critical process parameters. The guidelines aim to enhance product and process understanding using a science- and risk-based approach. This allows for greater control and consistency in manufacturing. The document also outlines regulatory and industry perspectives, as well as examples of applying QbD and modeling techniques scientifically.