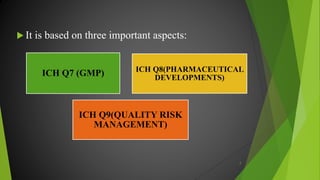
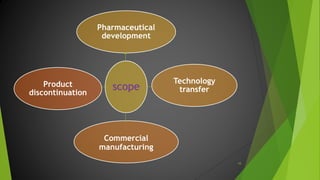
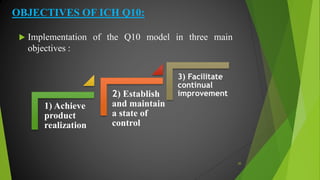
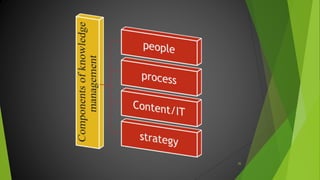
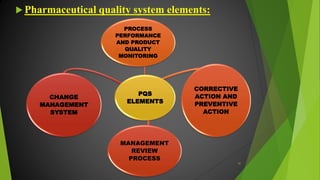
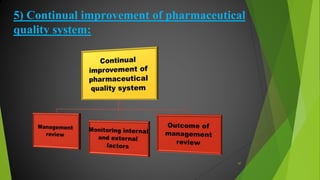
The ICH Q10 guideline outlines a comprehensive pharmaceutical quality management system to encourage a quality systems approach over traditional GMP compliance across the product lifecycle. It focuses on continuous improvement, management responsibilities, and establishes key objectives for product realization, control, and enhancement of quality. The effective implementation of this system involves knowledge management and quality risk management as integral components for ensuring the safety, efficacy, and quality of pharmaceuticals.