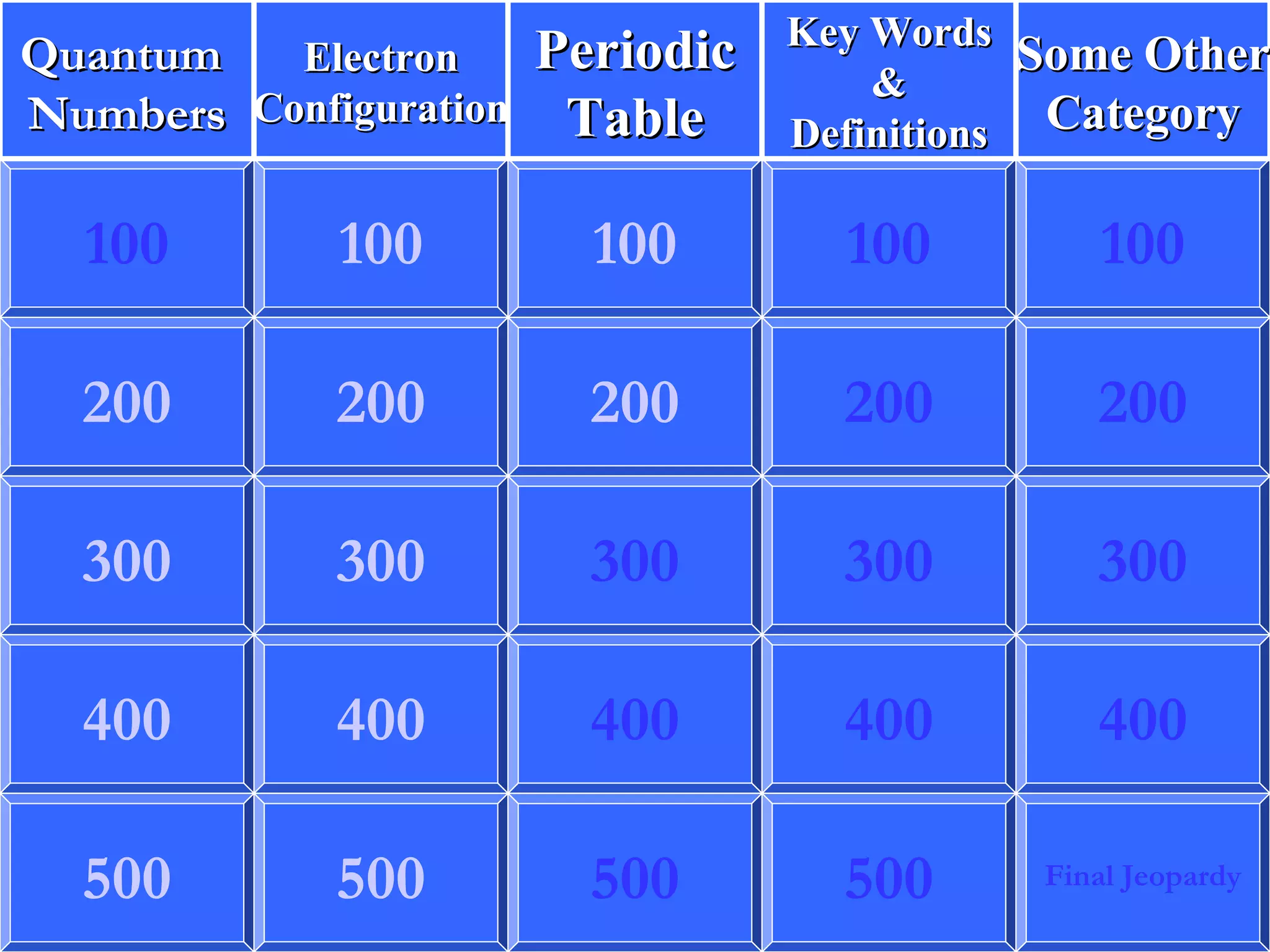
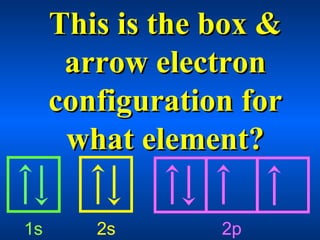
This document contains questions and answers about various topics in chemistry including atomic orbitals, electron configurations, quantum numbers, periodic table, ionization energy, and more. The questions cover definitions, properties, and examples related to these core concepts.
















![[Ar]4s[Ar]4s11
3d3d55](https://image.slidesharecdn.com/qnumbeconfigptjeopardy-101130033832-phpapp01/85/Qnumb-econfig-pt-jeopardy-19-320.jpg)

![[Uuo[Uuo118118
]8s]8s22
5g5g1818
6f6f1414
7d7d1010
8p8p33](https://image.slidesharecdn.com/qnumbeconfigptjeopardy-101130033832-phpapp01/85/Qnumb-econfig-pt-jeopardy-21-320.jpg)