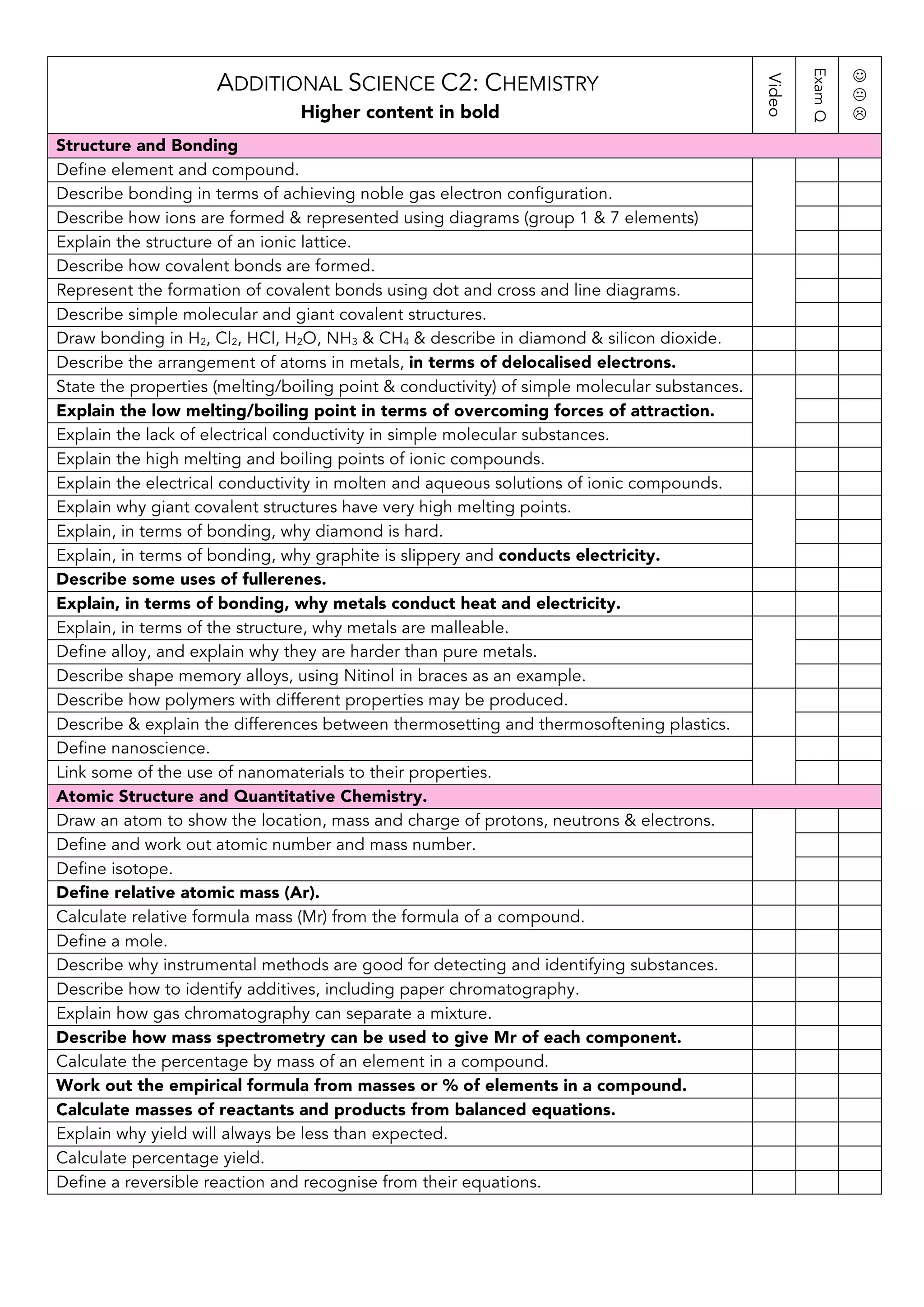
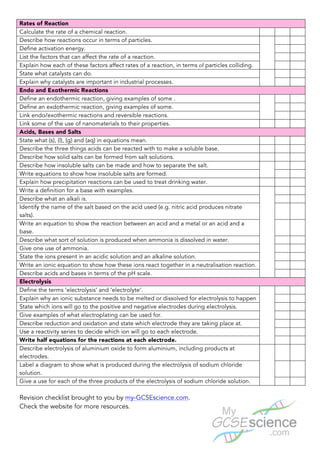
This document provides an overview of the content covered in additional science C2: chemistry. It outlines several key topics in chemistry including structure and bonding, atomic structure and quantitative chemistry, rates of reaction, endo/exothermic reactions, acids bases and salts, and electrolysis. For each topic, it lists the main concepts, definitions, and processes that students are expected to understand at a higher level, such as describing bonding using diagrams, calculating relative formula mass, explaining how factors affect reaction rates, writing equations for acid-base reactions, and describing electrolysis processes and products. The document serves as a revision checklist for students to ensure they have learned the essential high-level information and skills for the additional chemistry content.