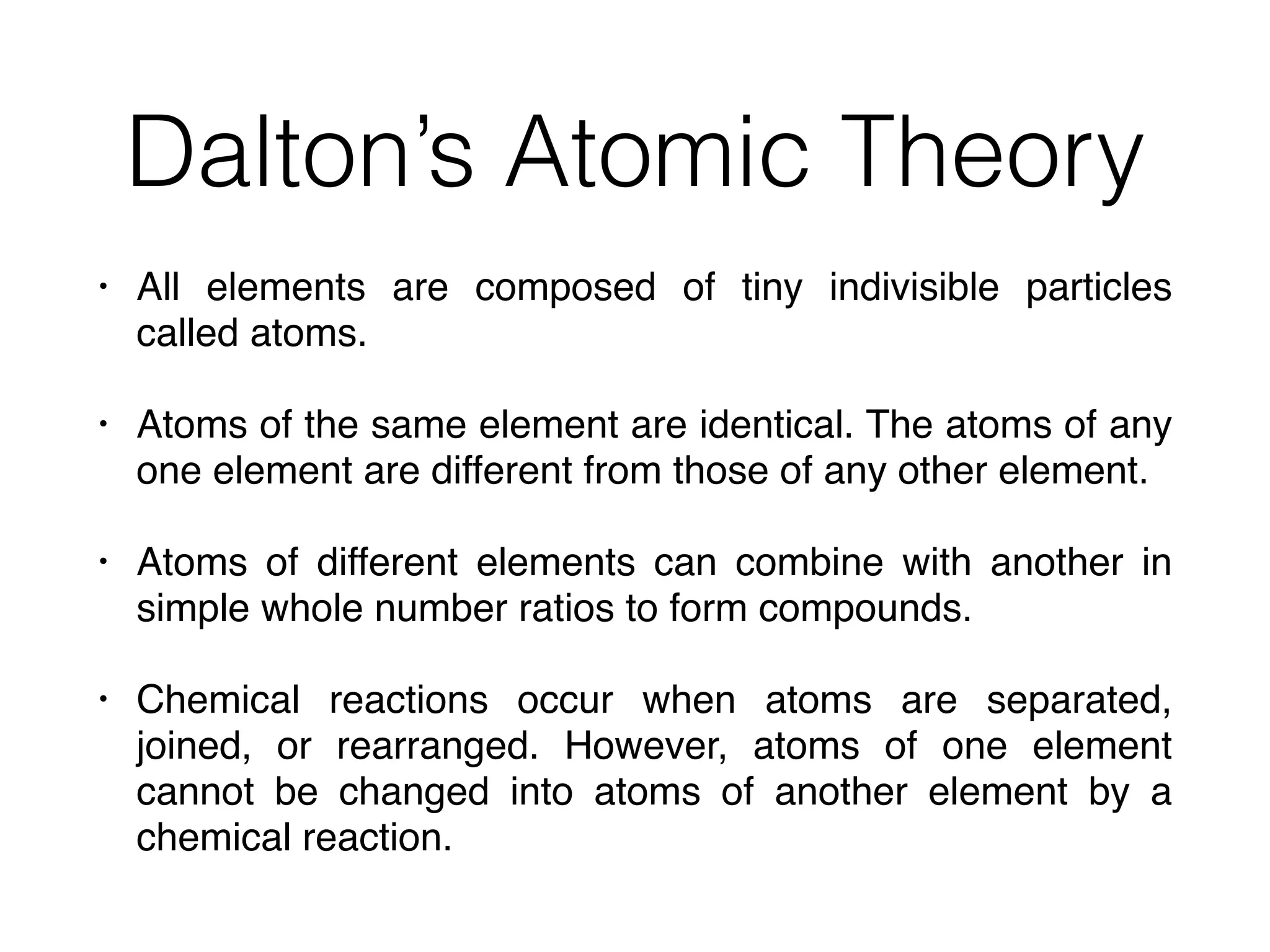
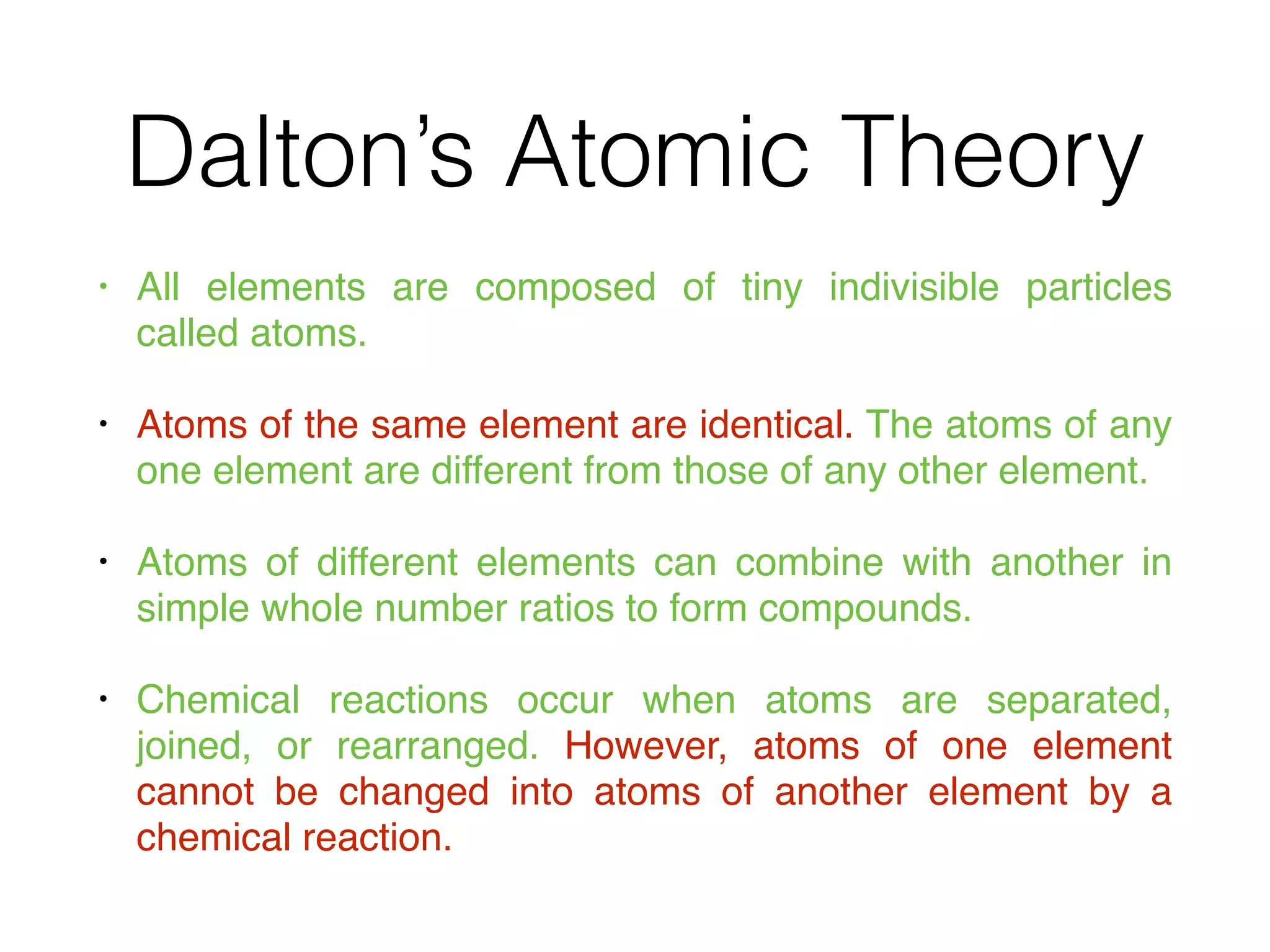
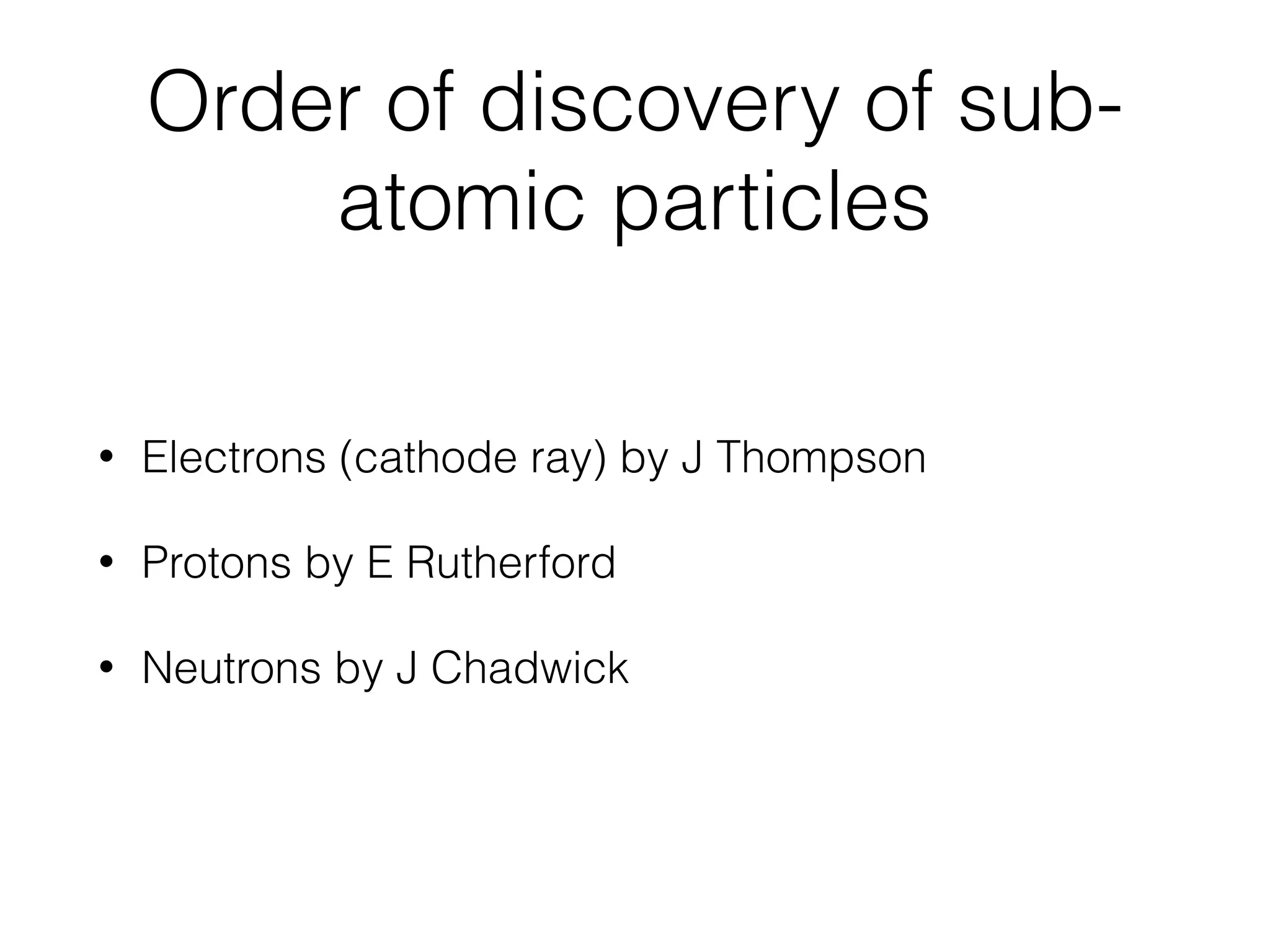
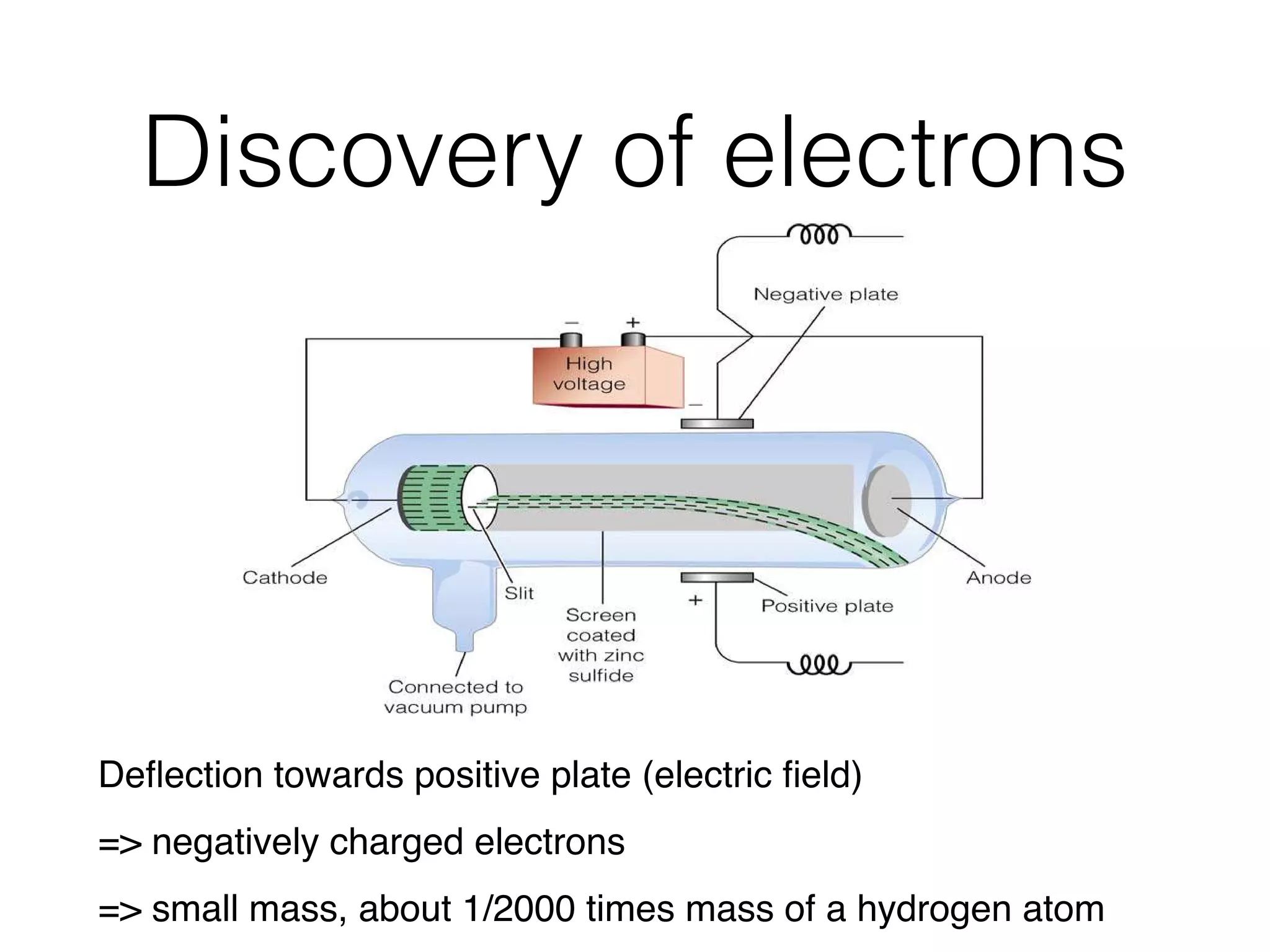
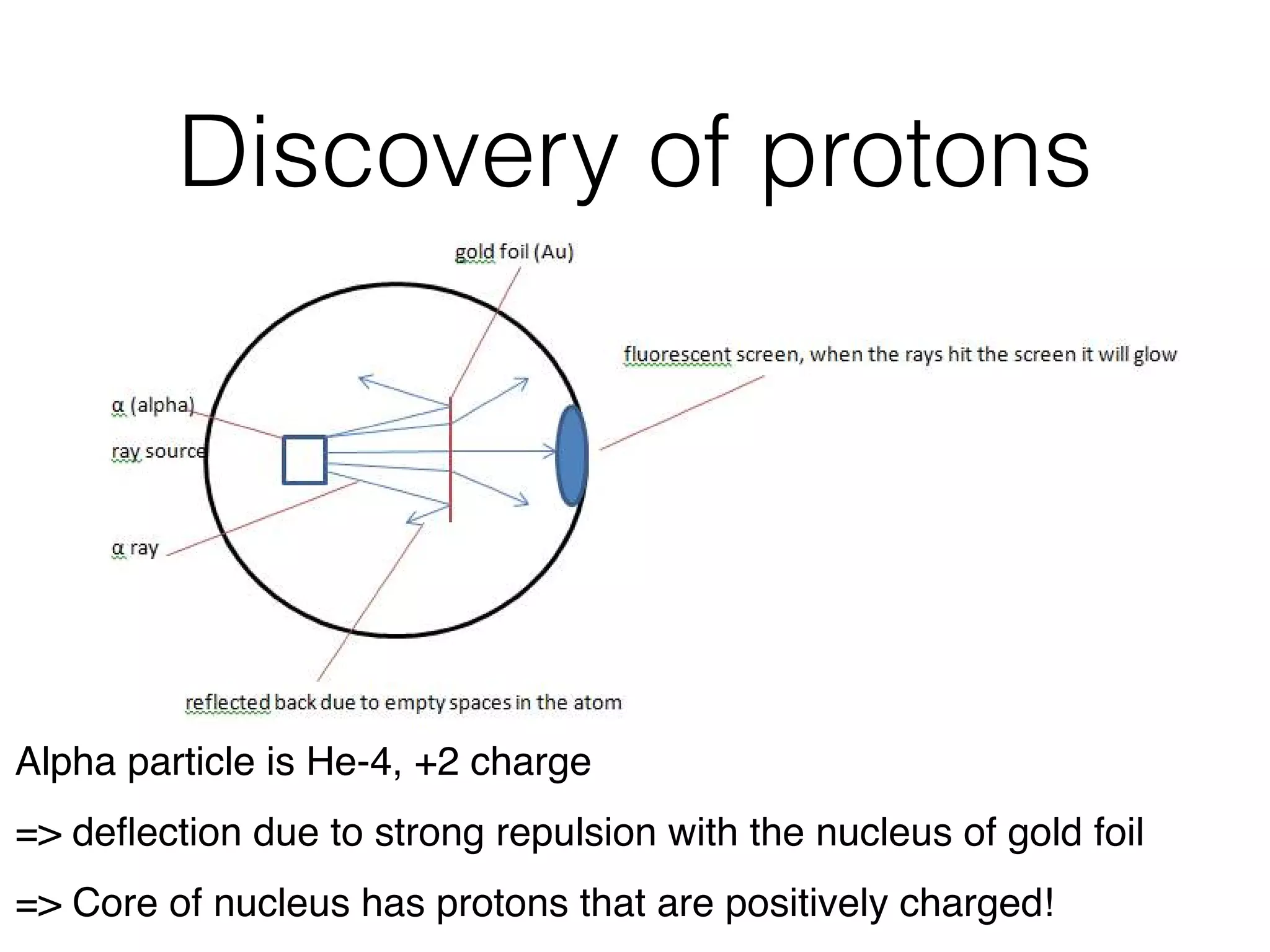
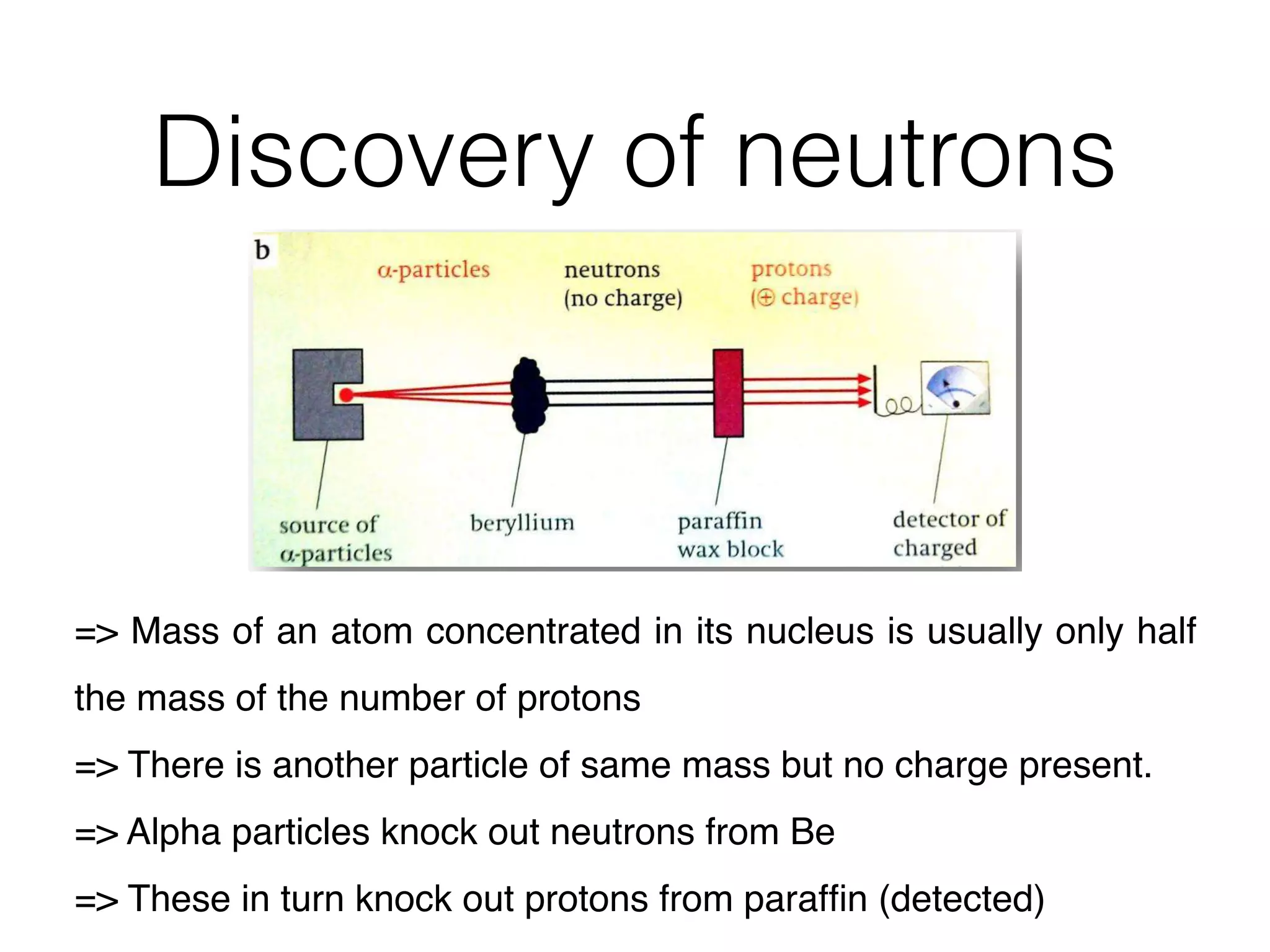
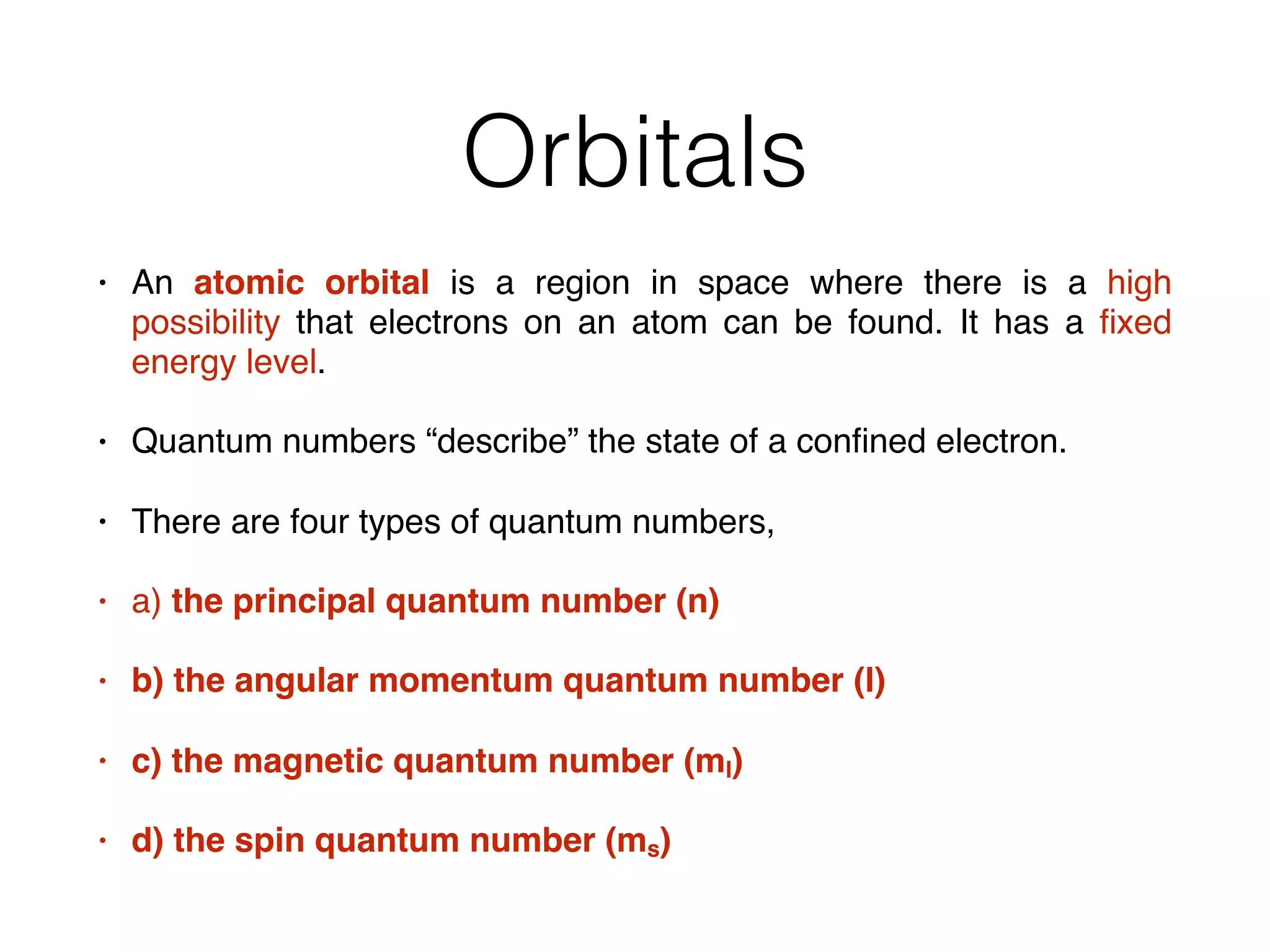
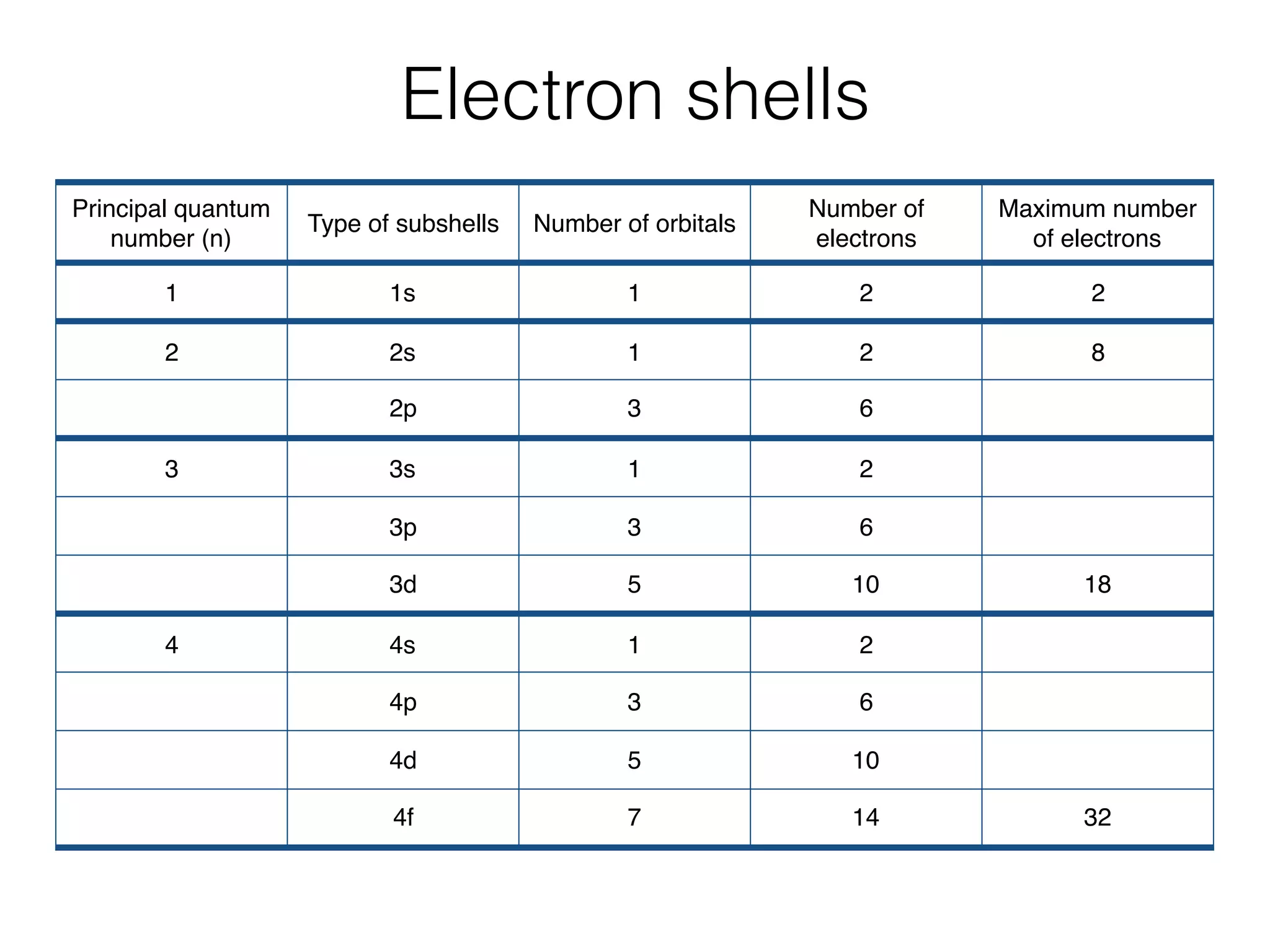
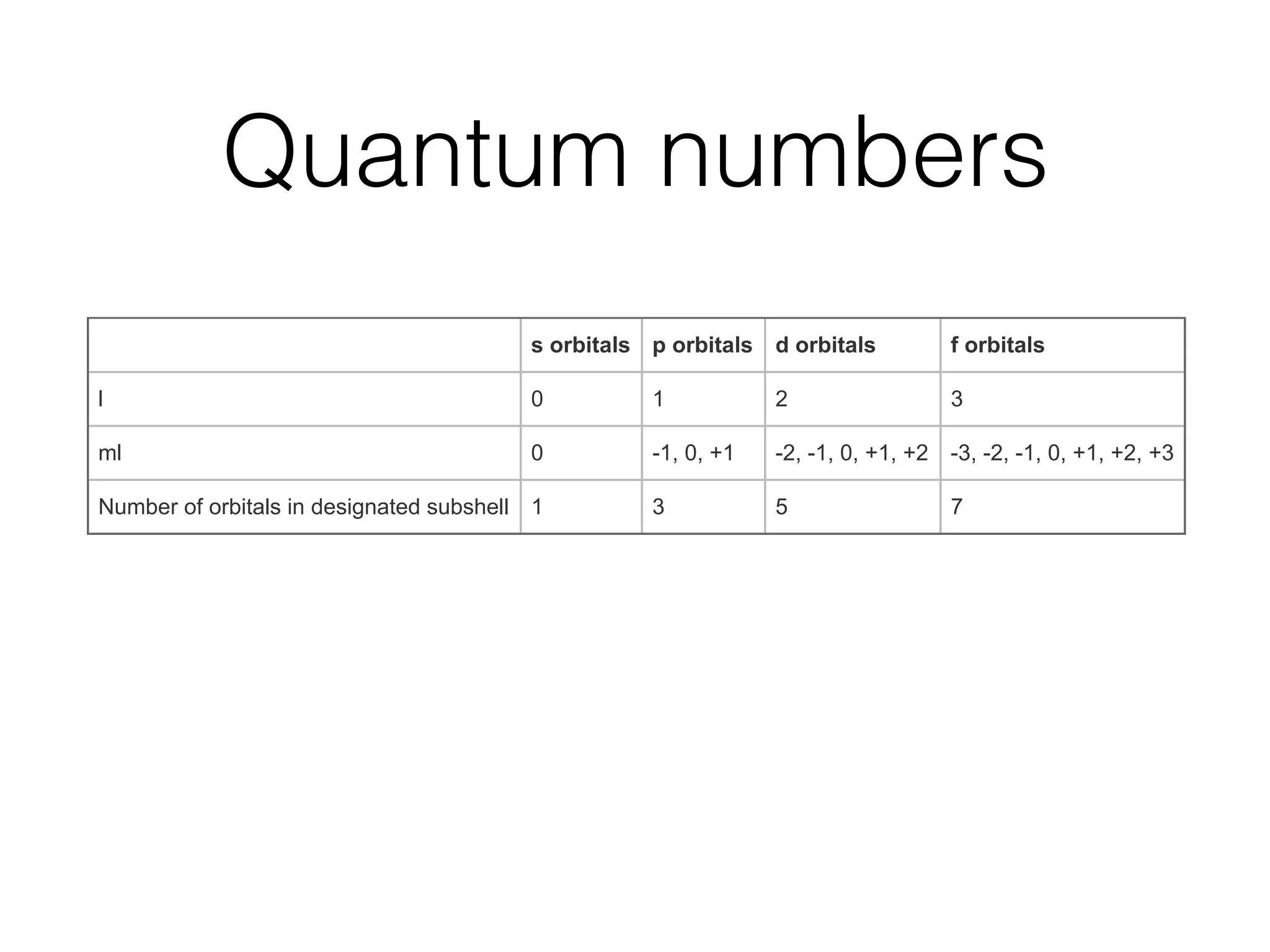
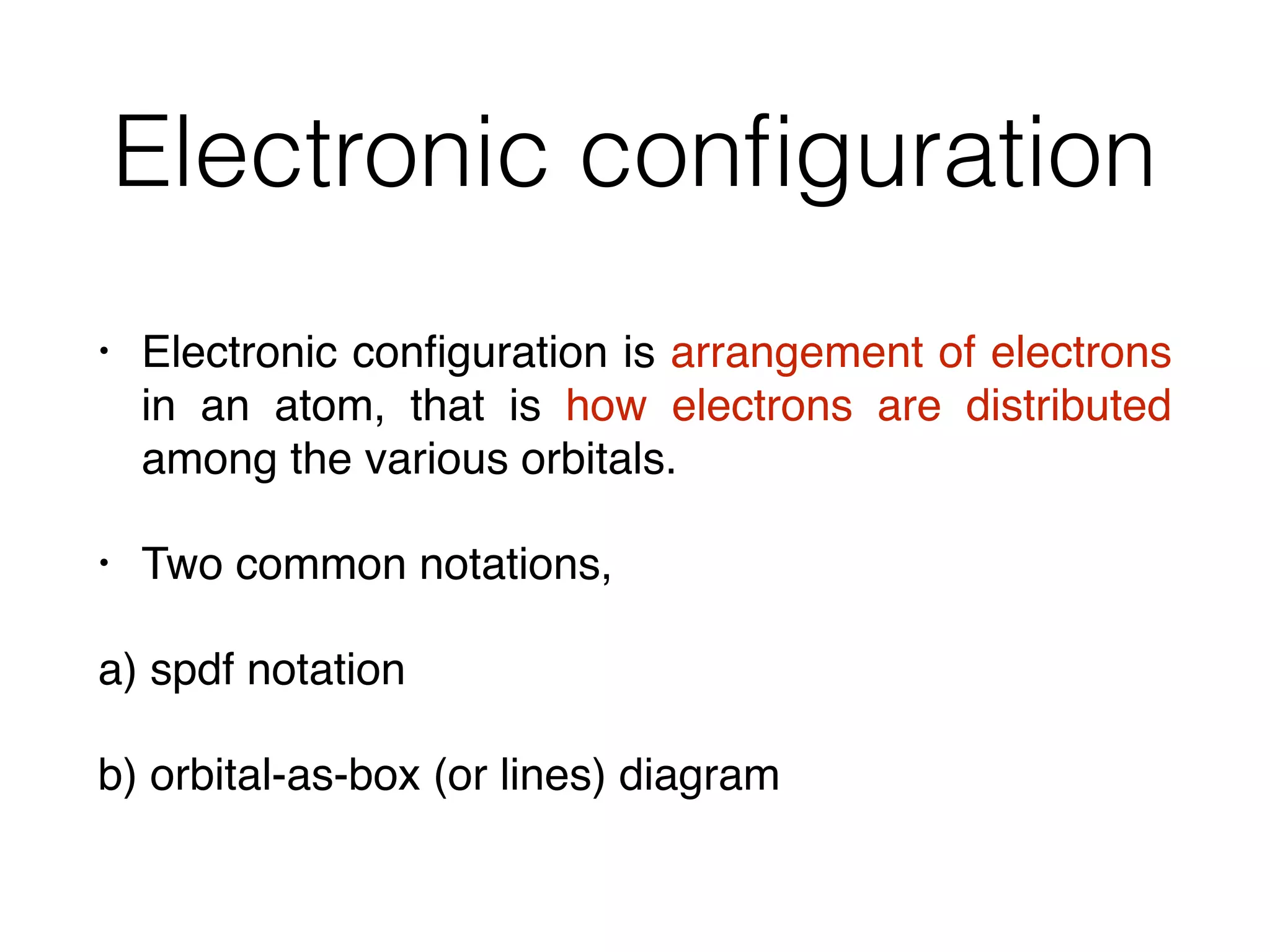
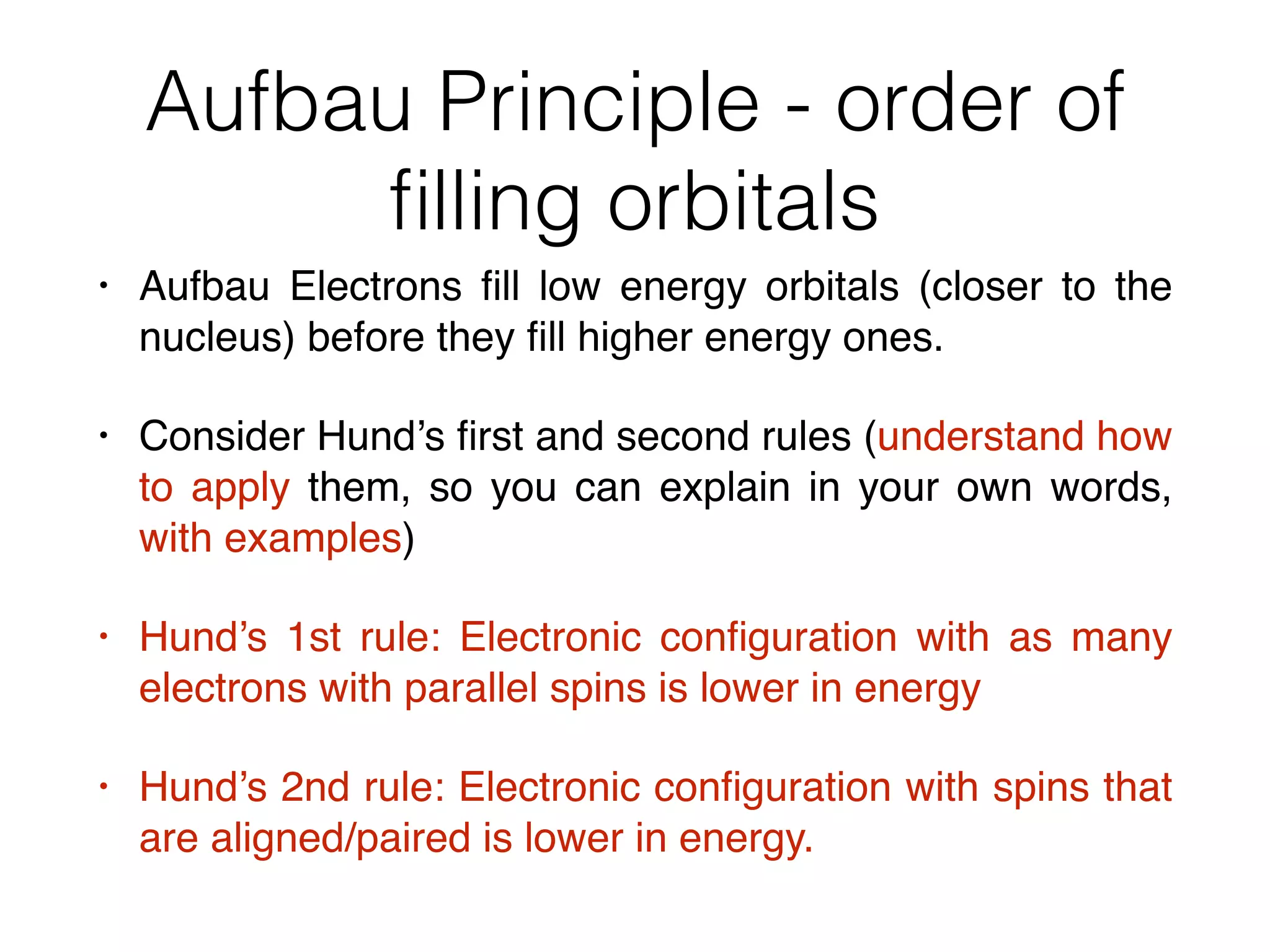
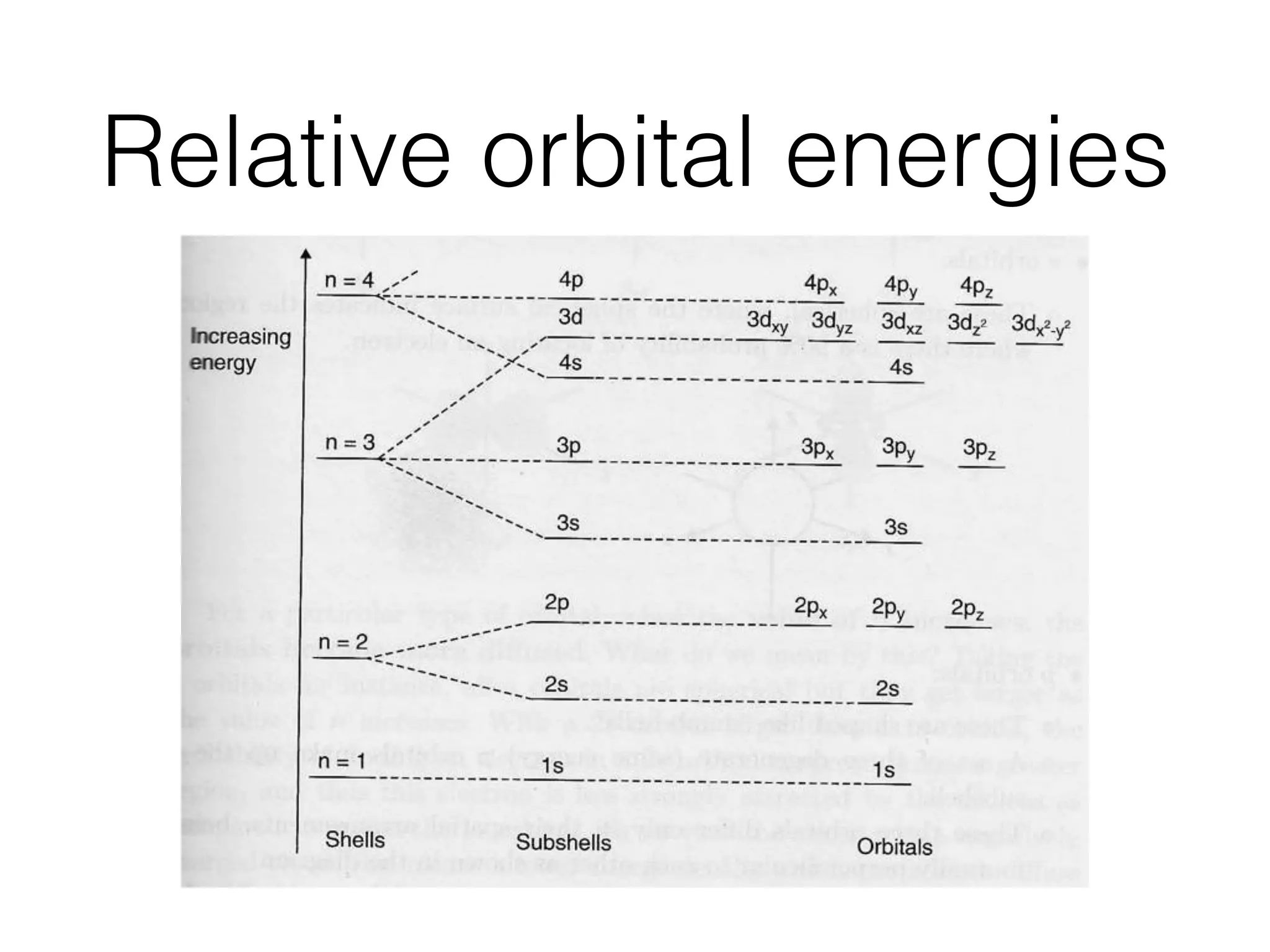
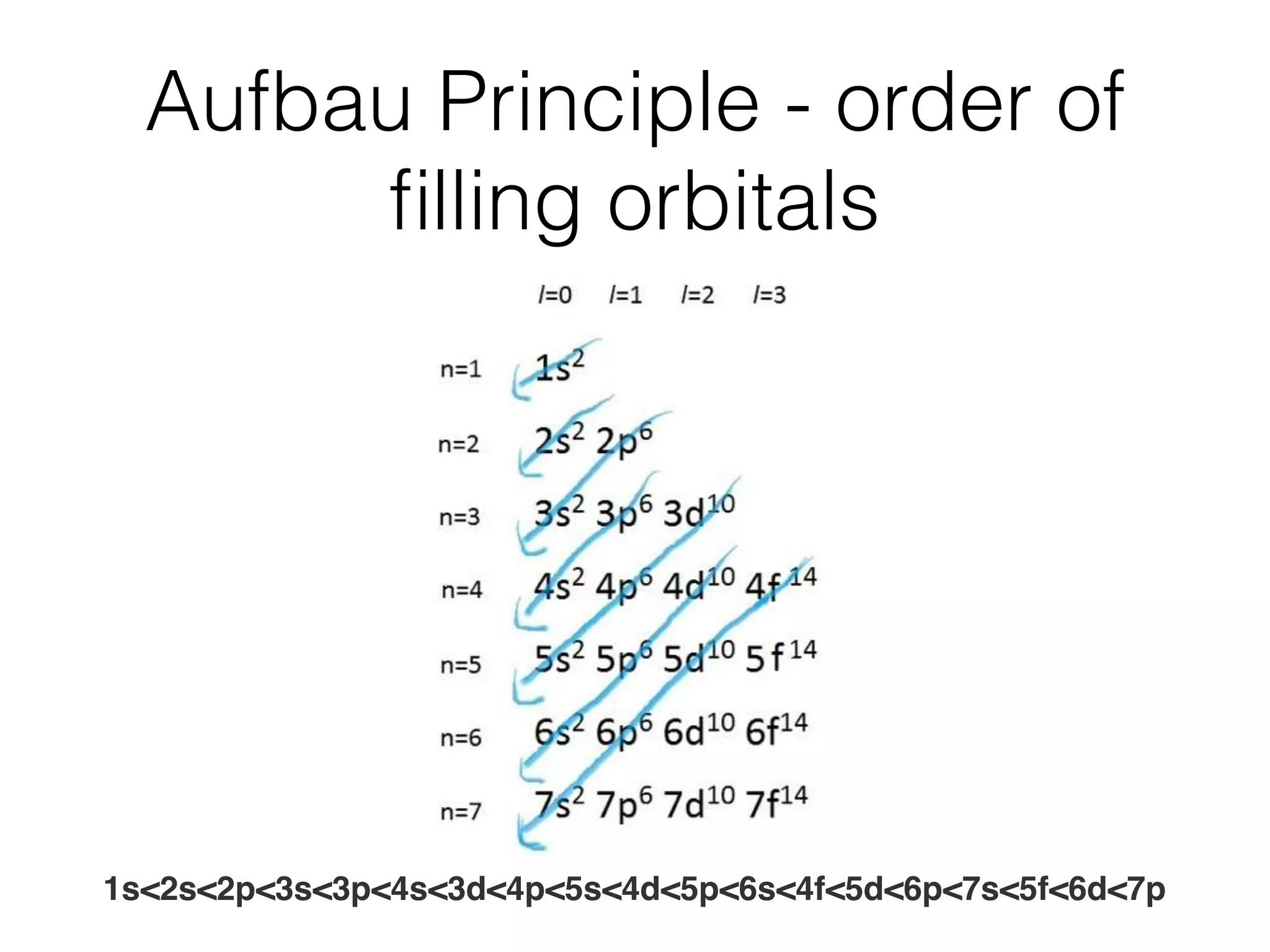
The document summarizes Dalton's atomic theory and provides information about atomic structure and subatomic particles. It discusses Dalton's four main postulates, including that atoms are indivisible and atoms of different elements combine in whole number ratios. The document also outlines the discoveries of key subatomic particles like electrons, protons, and neutrons by scientists such as Thomson, Rutherford, and Chadwick. It describes Bohr's model of the atom and introduces concepts like orbitals, electron configuration, and quantum numbers.





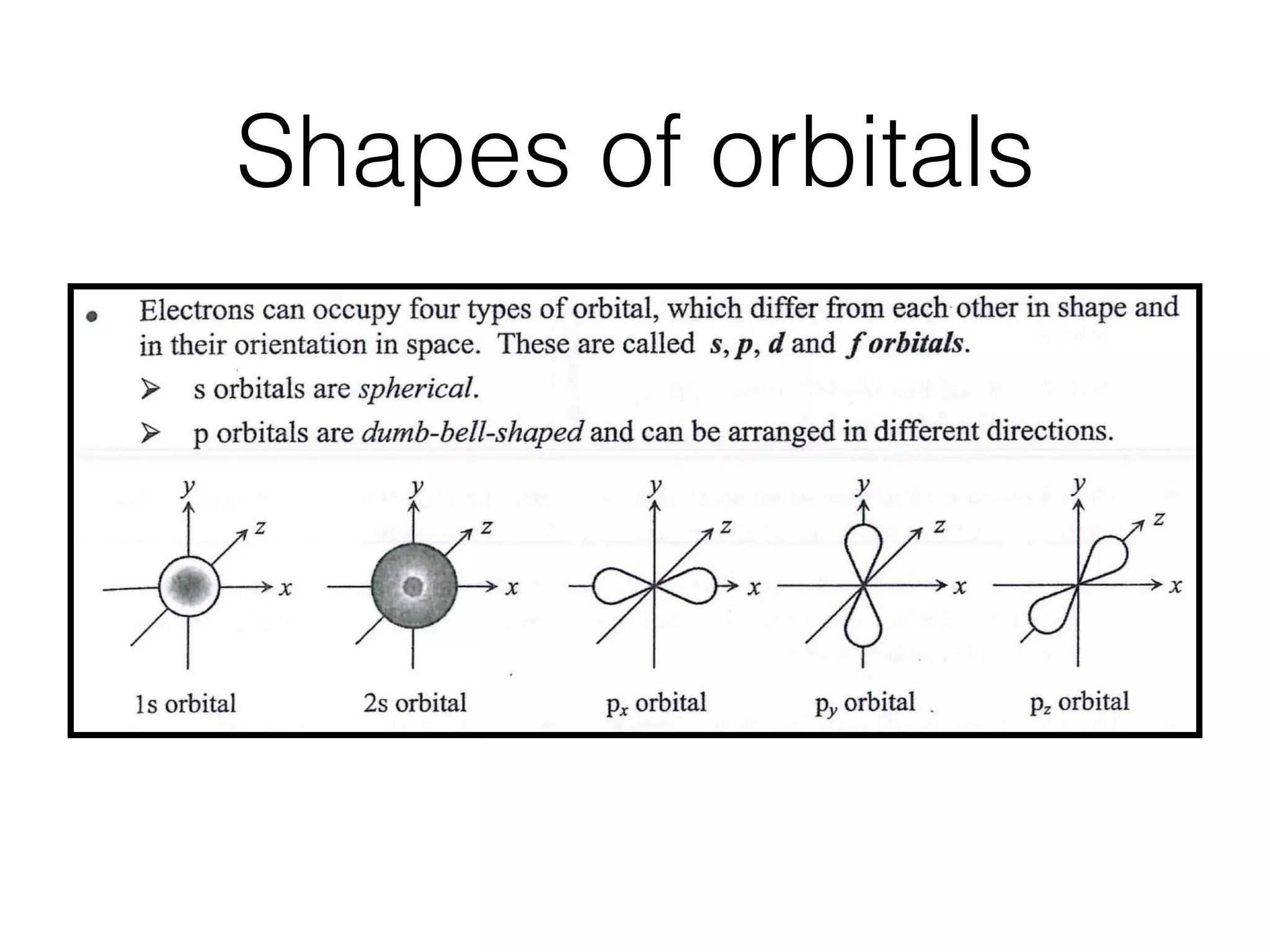
![Shapes of orbitalsl s orbital 2s orbital Px orbital Pr orbital . Pz orbital
There are five types of d orbitals (dxy, dye, d=, dx2_r, and dz, ).
Z Z ...-""! Z Z Z
Y .
/l~/'~': Y
y
.:::.ii
~ / i . >x
' ~
"~ X
X X
: .~
dxy orbital v- d. orbital d= orbital dx,_y~ orbital d~, orbital
Each of d~y, dy~ and d= orbitals consists of four lobes (of the same size and
same shape) on the xy, yz and zx plane respectively.
The d~2_?2 orbital consists of four lobes along the x- and y- axes.
The shape of the dz, orbital is different from the other four- it consists of two
lobes along the z-axis with a 'ring' in the middle.
All the d orbitals are degenerate; i.e. of the same energy level.
[NB. In drawing shapes of orbitals, the x-, y- and z- axes must be shown so as to
illustrate the 3-D property of the orbitals.]
Shells a](https://image.slidesharecdn.com/csonnt2atomicstructure-160121035627/75/Csonn-t2-atomic-structure-18-2048.jpg)


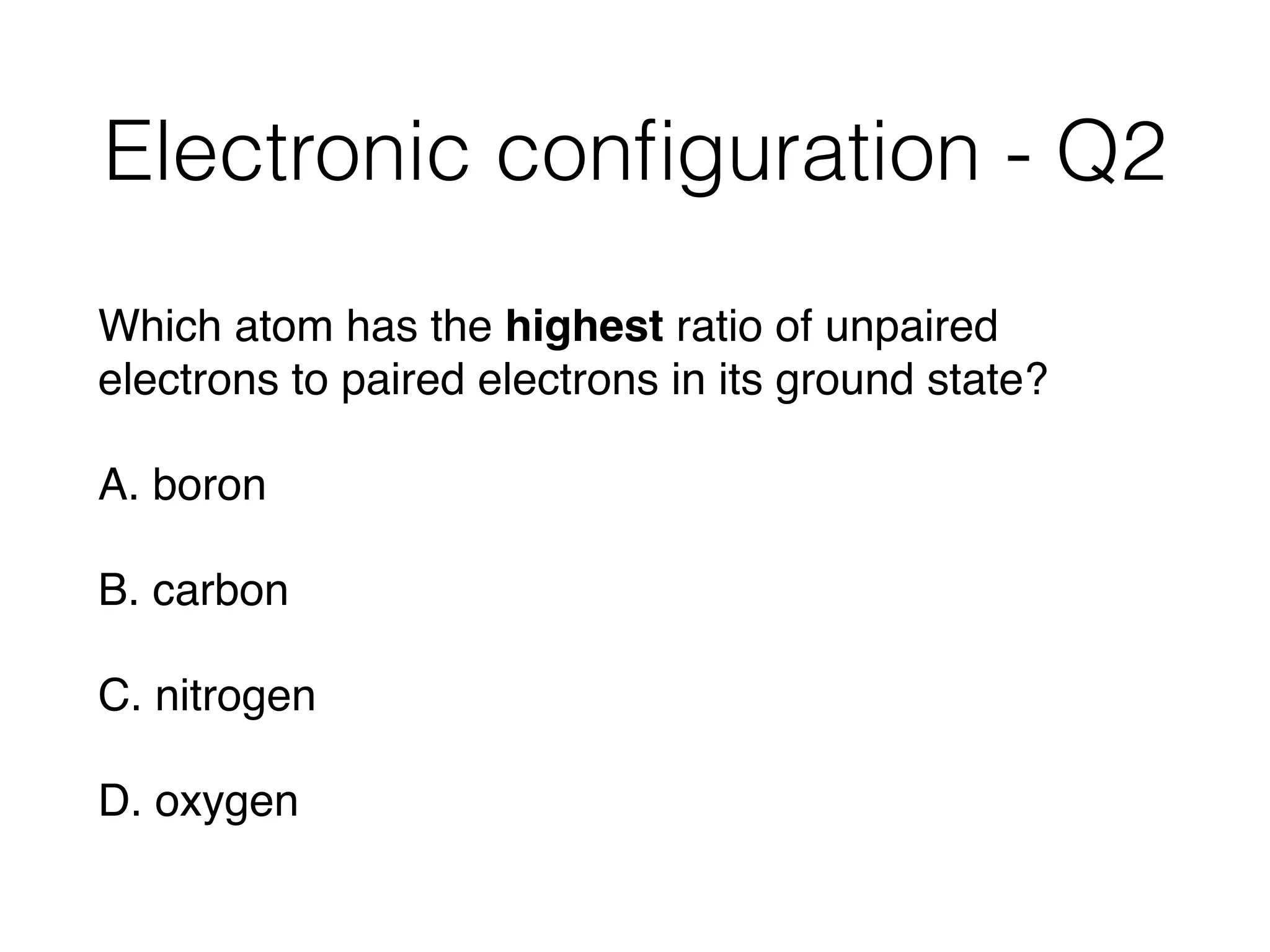
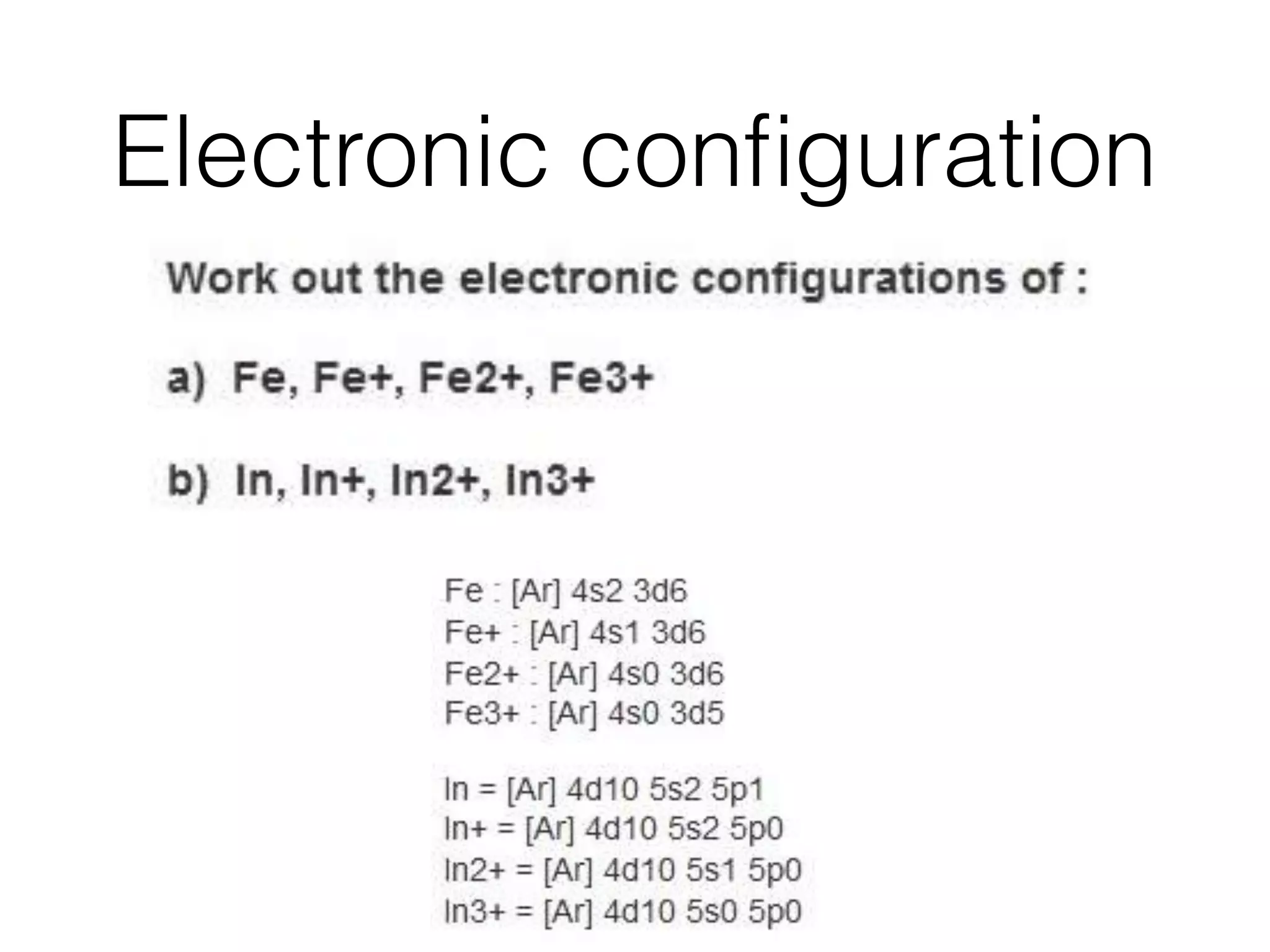
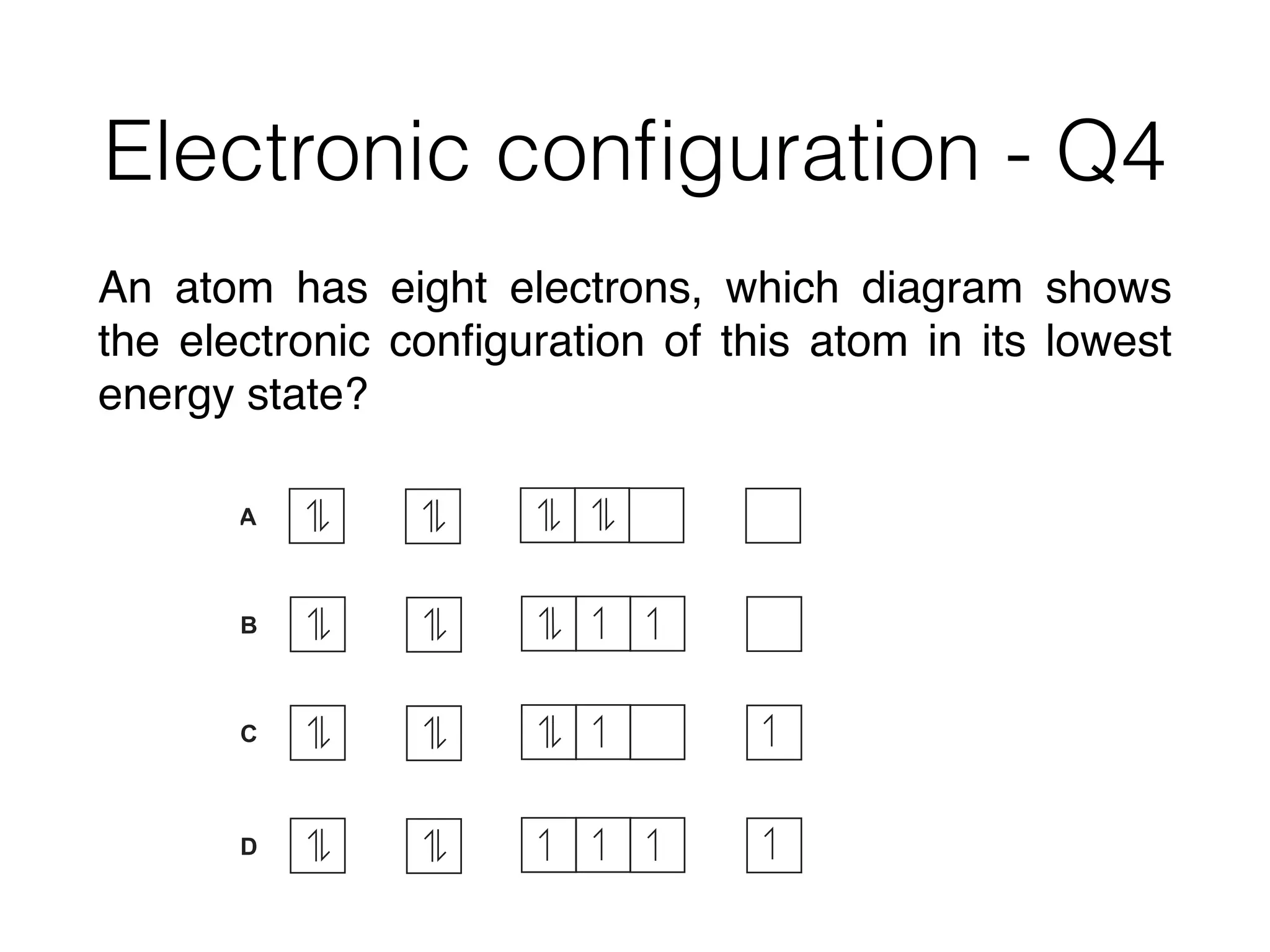
![Electronic configuration - Q3
An ion of manganese has an electronic configuration
of [Ar] 3d4. Which compound contains this ion?
A. MnCl2 B. MnO C. Mn2O3 D. MnO2](https://image.slidesharecdn.com/csonnt2atomicstructure-160121035627/75/Csonn-t2-atomic-structure-33-2048.jpg)










I , 1
2 ', ! (3 quantum shell) ', ',
i ! ',
1 ~ ' t , , , ~ , , I ~ , , , , ~ , i ~ ~ J
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
order of electrons removed
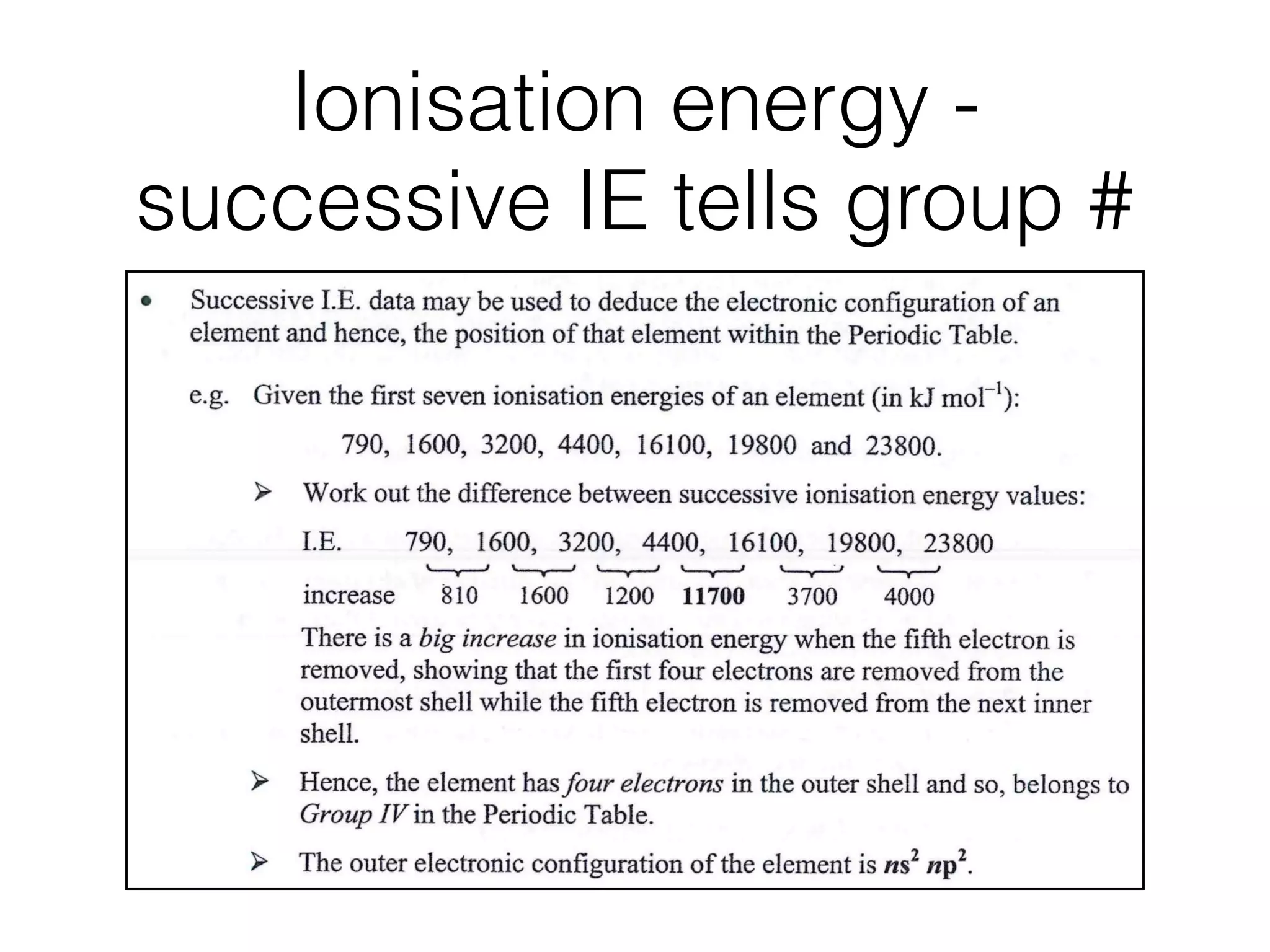
Potassium atom has a total of 19 electrons, which fall into four groups.
- two electrons very close to the nucleus (in the 1st quantum shell, which are
most difficult to remove),
- eight electrons further out (in the 2na quantum shell),](https://image.slidesharecdn.com/csonnt2atomicstructure-160121035627/75/Csonn-t2-atomic-structure-53-2048.jpg)
I , 1
2 ', ! (3 quantum shell) ', ',
i ! ',
1 ~ ' t , , , ~ , , I ~ , , , , ~ , i ~ ~ J
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
order of electrons removed
Potassium atom has a total of 19 electrons, which fall into four groups.
- two electrons very close to the nucleus (in the 1st quantum shell, which are
most difficult to remove),
- eight electrons further out (in the 2na quantum shell),
- another eight electrons even further out (in the 3rd quantum shell) and
- one further away still (in the 4th quantum shell).
Hence, the electron arrangement in potassium is written as 2,8,8,1.
Ionisation energies in the 3ra quantum shell of potassium
The steady rise in ionisation energy for
successive removal of the first six electrons
followed by a sharp increase, suggests that the
last two electrons are more strongly attracted
lg I.E.](https://image.slidesharecdn.com/csonnt2atomicstructure-160121035627/75/Csonn-t2-atomic-structure-54-2048.jpg)
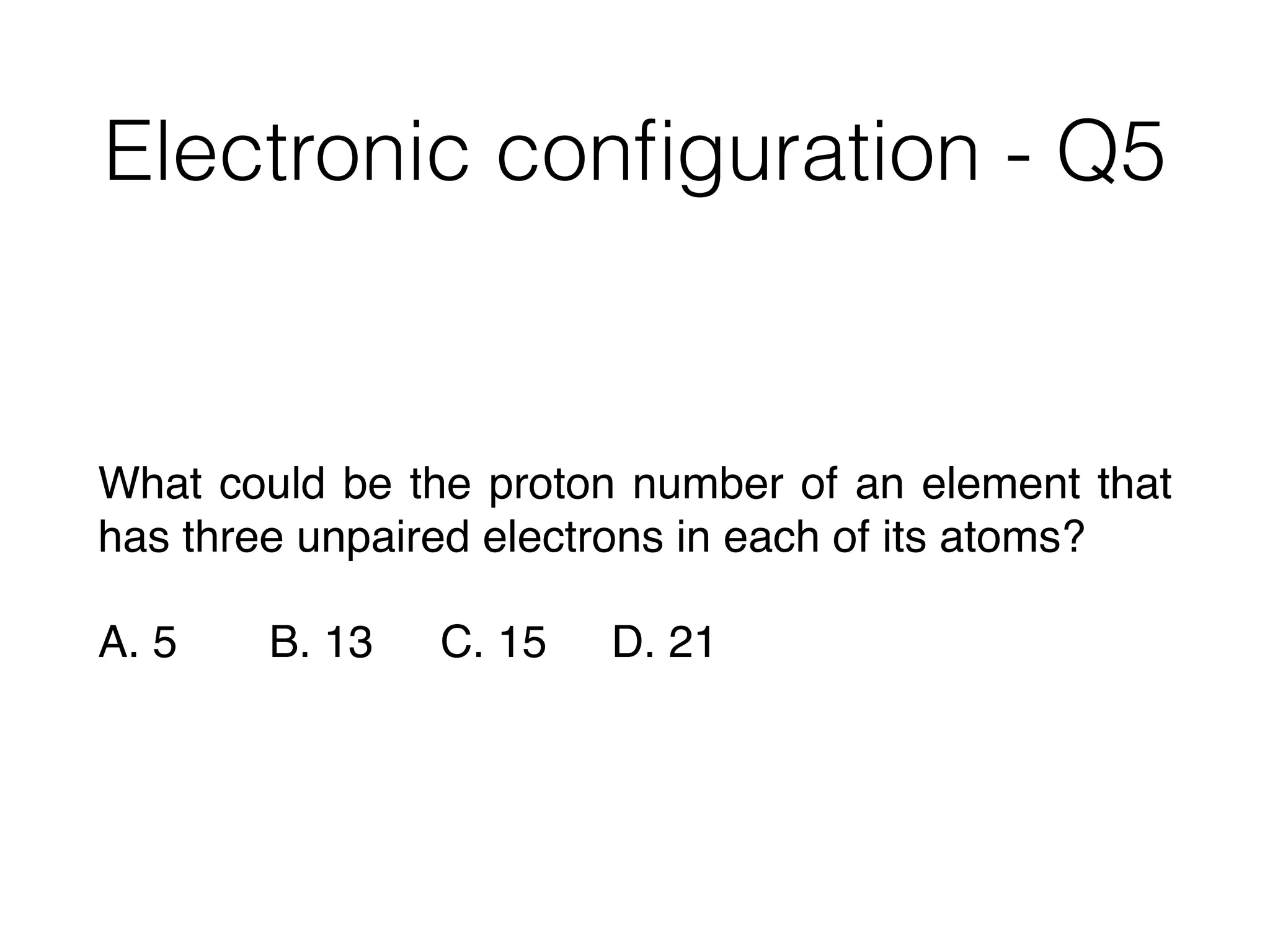
![Successive ionisation
energies - Q4
2
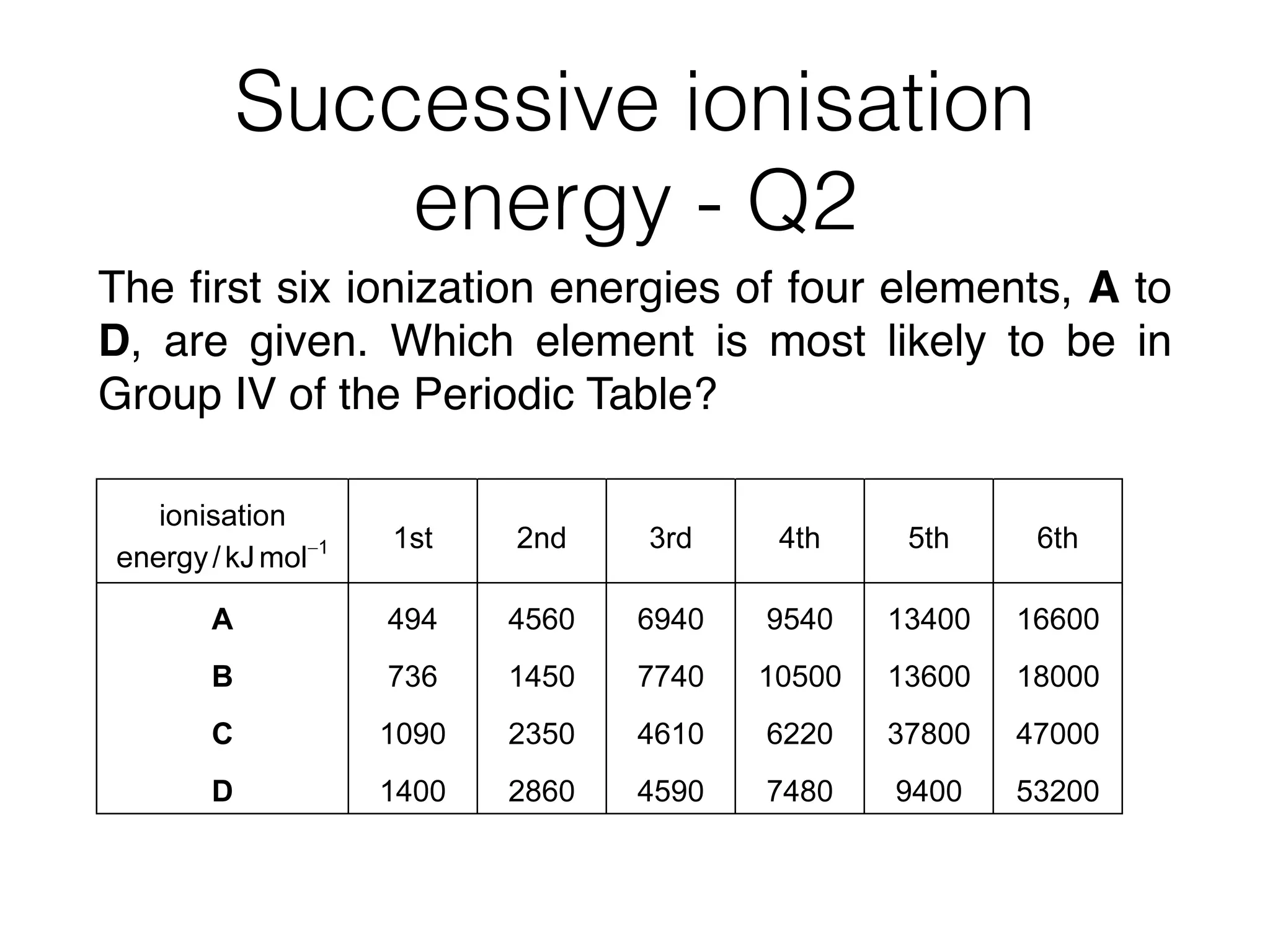
1 The table shows the successive ionisation energies for an element Q.
1st 2nd 3rd 4th
ionisation energy/kJmol–1
418 3070 4600 5860
What is the likely formula of the oxide of Q?
A QO B Q3O2 C Q2O D Q2O3
2 How many neutrons are present in 0.13g of 13
C?
[L = the Avogadro constant]
A 0.06L B 0.07L C 0.13L D 0.91L](https://image.slidesharecdn.com/csonnt2atomicstructure-160121035627/75/Csonn-t2-atomic-structure-58-2048.jpg)