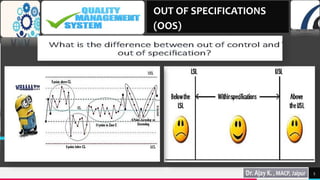
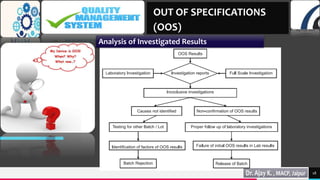
This document discusses out of specification (OOS) results and the processes for investigating them. It covers:
1) What OOS is and when investigations are conducted.
2) The initial laboratory investigation and the responsibilities of the analyst and supervisor.
3) Full-scale investigations which include reviewing manufacturing, production, sampling, and initial lab results.
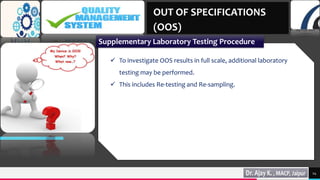
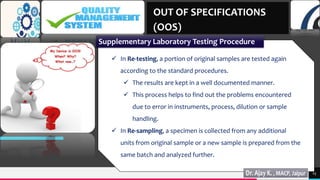
4) Supplementary laboratory testing like re-testing and re-sampling to identify the source of errors.
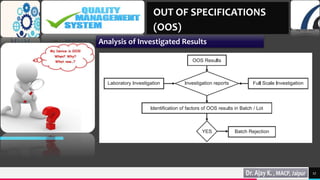
5) Analyzing the investigated results to determine the possible causes of OOS results.