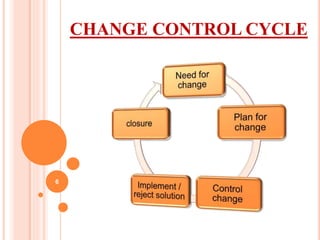
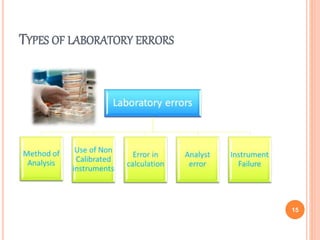
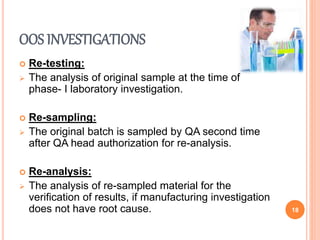
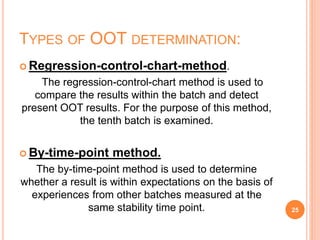
This document discusses concepts of change control, out of specifications (OOS), and out of trends (OOT) in pharmaceutical quality assurance. It defines change control as a procedure to review, verify, regulate, manage, approve and control changes made to systems or processes. OOS refers to test results that fall outside pre-defined acceptance criteria, while OOT describes results that do not follow expected trends. The document outlines procedures for investigating and managing changes, OOS, and OOT to ensure product quality and compliance with regulations.