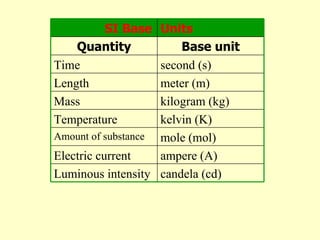
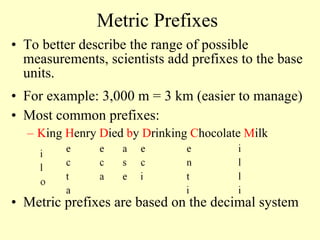
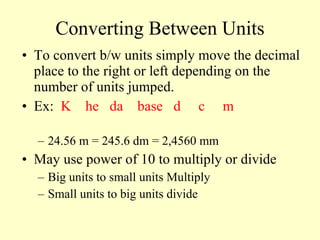
1. The document discusses units of measurement and the SI system. It describes the seven base SI units including meters, kilograms, seconds, and kelvins.
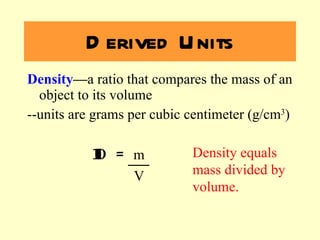
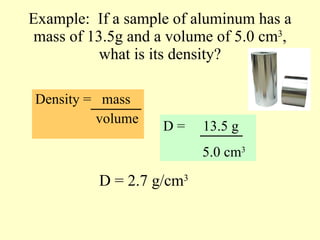
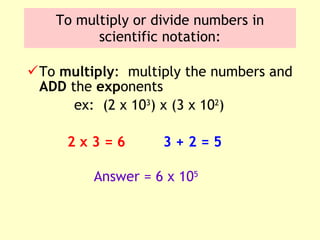
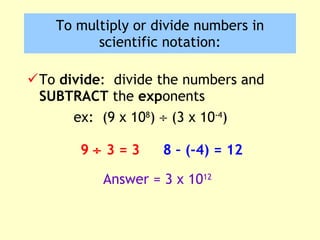
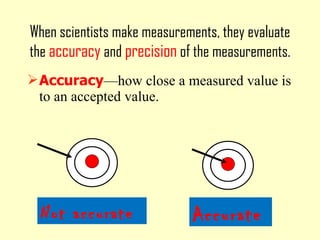
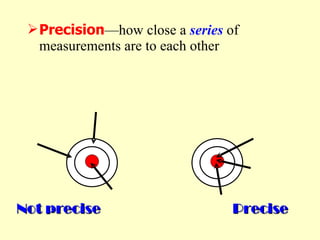
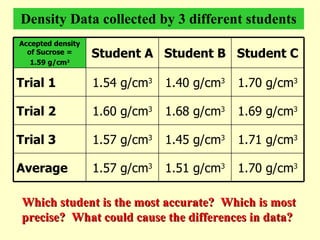
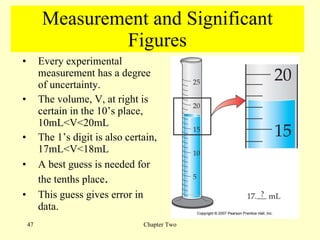
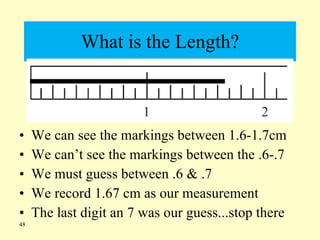
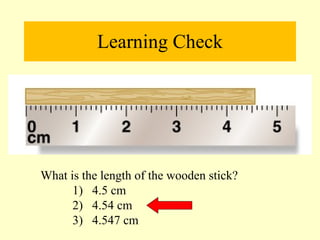
2. Derived units are discussed along with examples like density. Significant figures and the accuracy and precision of measurements are also covered.
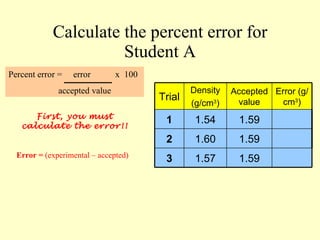
3. Errors in measurements are defined as the difference between experimental and accepted values. Percent error can quantify the accuracy of a measurement.