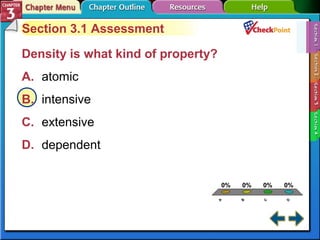
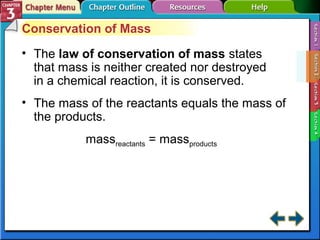
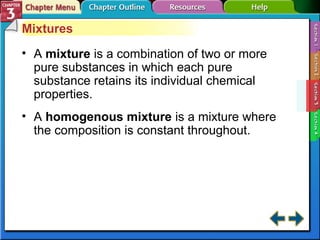
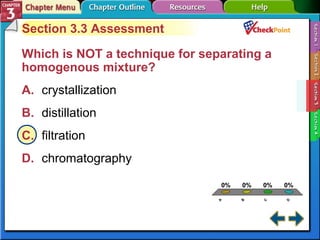
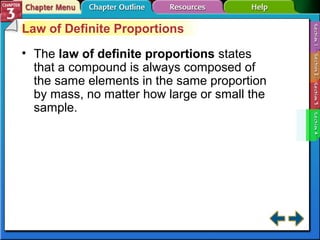
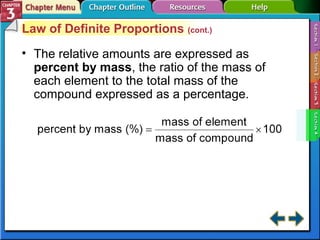
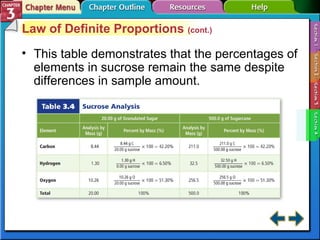
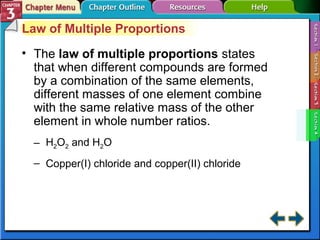
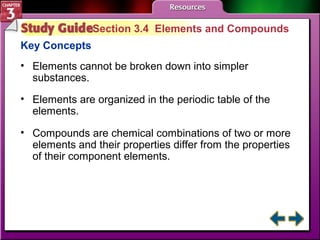
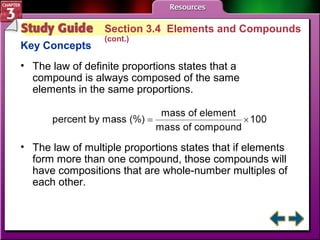
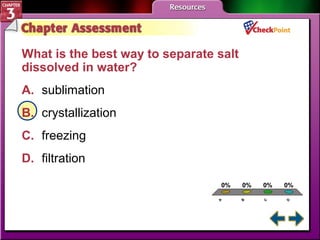
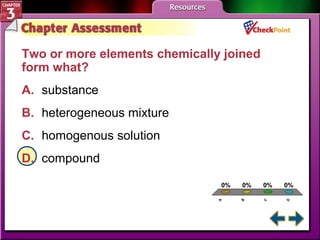
This document provides an overview of key concepts from a chemistry textbook chapter on matter, including its properties, changes, mixtures, and elements/compounds. It discusses the three states of matter and defines physical and chemical properties. It also describes two types of changes matter can undergo (physical vs. chemical) and techniques for separating mixtures. Finally, it defines elements and compounds, and explains how they are organized in the periodic table according to atomic structure. The chapter assessments cover identifying properties, states of matter, and distinguishing between mixtures, elements and compounds.








































![Click any of the background top tabs
to display the respective folder.
Within the Chapter Outline, clicking a section
tab on the right side of the screen will bring you
to the first slide in each respective section.
Simple navigation buttons will allow you to
progress to the next slide or the previous slide.
The Chapter Resources Menu will allow you to
access chapter specific resources from the Chapter
Menu or any Chapter Outline slide. From within any
feature, click the Resources tab to return to this
slide.
The “Return” button will allow you to return to the
slide that you were viewing when you clicked either
the Resources or Help tab.
To exit the presentation, click the Exit button on the Chapter Menu slide or
hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide.](https://image.slidesharecdn.com/cmcchapter03-141003120148-phpapp02/85/chemistry-chapter-03-all-sections-in-one-powerpoint-74-320.jpg)
