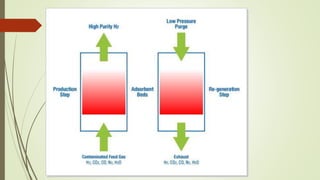
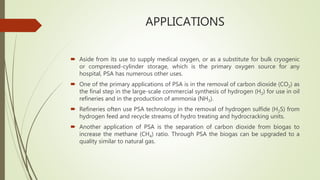
Pressure swing adsorption is a process that uses selective adsorption to separate gas mixtures. It works by passing a gas mixture through an adsorbent bed that attracts some gases more than others under pressure. When pressure is reduced, the adsorbed gases are released. Using two beds and alternating pressures allows for continuous gas production. Common adsorbents like zeolites and activated carbon can separate gases like oxygen from air or hydrogen sulfide from hydrocarbon streams. Pressure swing adsorption has various industrial applications such as oxygen production, hydrogen purification, and nitrogen generation.