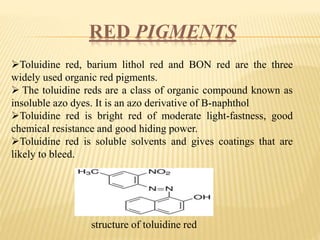
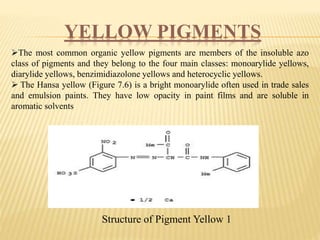
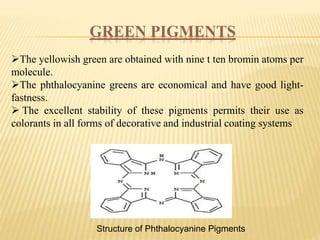
This document discusses pigments used in paint formulations. It covers the main functions of pigments which are providing color, opacity, and protection. It discusses the particle size of pigments and how they refract light. It also covers important properties like hiding power, tinting strength, refractive index, light fastness, bleeding characteristics, and particle size and shape. The document classified pigments by origin, chemical structure, and as food additives. It provides details on important types of pigments including organic pigments, white pigments like titanium dioxide, colored pigments like red iron oxide, yellow and orange pigments, and blue and green pigments. It also briefly discusses metallic pigments.