This document discusses vaporizers, which are devices used to convert liquid anesthetic agents into vapor for delivery to patients. It covers the basic principles of vaporization, factors that affect vaporizer output concentration, different types of vaporizers classified by their design and operating characteristics, and standards for vaporizer design. The key points are that vaporizers precisely regulate anesthetic vapor concentrations, multiple factors influence output, and designs vary in things like temperature compensation, agent specificity, and positioning within the breathing circuit.

![1.DO YOU FEEL YOU NEED IT?2.SOME IMPORTANT THINGS!3.CLASSIFICATION4.FACTORS AFFECTING VAPORIZATION5.ASTM VAPORIZER STANDARDS6.FILLING SYSTEMS7.HAZARDS[lengthy class..is it a hazard?]8.EVOLUTION](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-2-320.jpg)


![SO VAPOR IS THE GASEOUS PHASE OF A SUBSTANCE BELOW ITS CRITICAL TEMPERATURELove’ [van der Waals forces] promotes the liquid state, whereas ‘hatred’ [ Kinetic energy] promotes vapor phase](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-5-320.jpg)

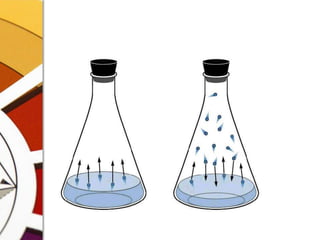

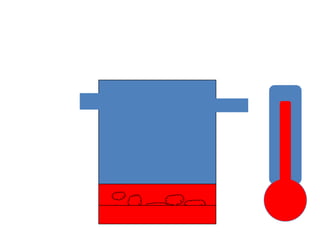
![Boiling PointYou go on increasing the temperatureThe V.P. will increaseAt a point it equals the atm pressureB.P.OF A LIQUID IS THE TEMPERATURE AT WHICH ITS VAPOR PRESSURE IS EQUAL TO THE ATM PRESSURELow atm pressure -> Low B.P.High SVP -> Low B.P.[..means I am very volatile]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-10-320.jpg)

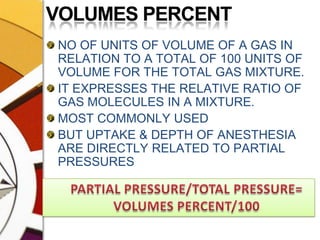












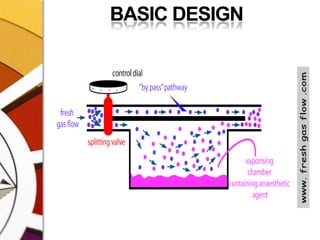

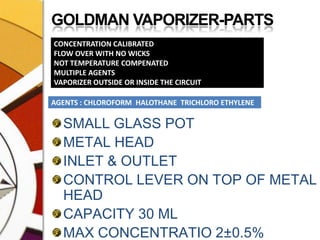
![CONCENTRATION CALIBRATEDTOTAL FLOW FROM THE MACHINE PASSES THROUGH THE VAPORIZER. THIS IS SPLIT BY A VARIABLE RESISTANCE PROPORTIONATING VALVE INTO TWO:ONE PART[USUALLY MAJOR] FLOWS THROUGH THE BYPASS CHAMBER & THE OTHER [USUALLY SMALL] THROUGH THE VAPORIZING CHAMBER.](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-32-320.jpg)

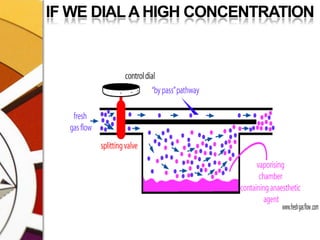
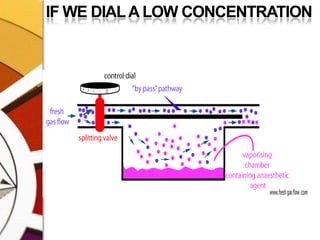





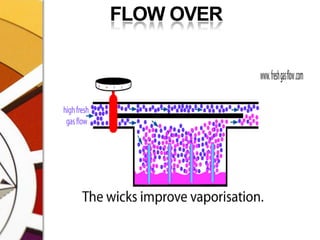

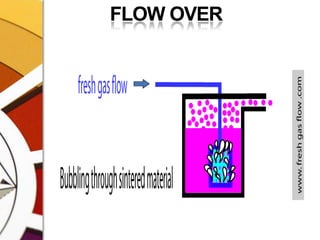



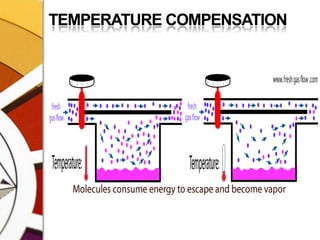
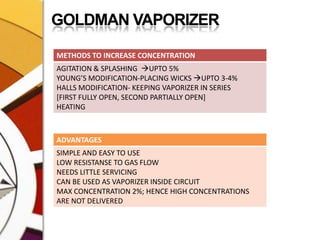
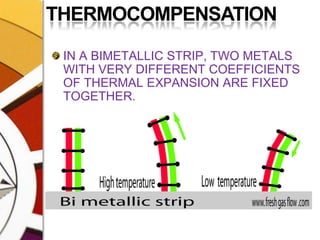
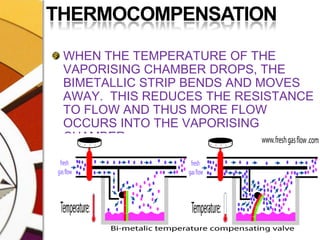
![THERMOCOMPENSATIONMOST VARIABLE BYPASS VAPORIZERS COMPENSATE FOR CHANGES IN VAPOR PRESSURE BY ALTERING THE SPLITTING RATIO.DONE BY USING A THERMOSENSITIVE ELEMENT [BIMETALLIC STRIP] INCORPORATED IN THE VAPORIZING CHAMBER OR BYPASS CHAMBER.SO, THE SPLITTING OF GAS IS CONTROLLED BY TWO VALVES: (1)THE DIAL WE SET (SPLITTING VALVE) AND (2)THE TEMPERATURE COMPENSATING VALVE](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-48-320.jpg)

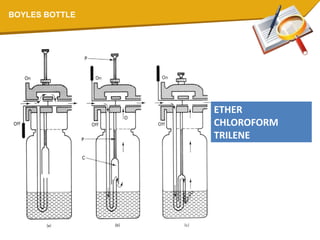
![PLENUM [Latin= fullness]VAPORIZERS WITH HIGH RESISTANCE WHICH DEPEND ON COMPRESSED GAS DRIVEN UNDER PRESSURE ARE CALLED PLENUM VAPORIZERS.e.g. BOYLE BOTTLE,COPPER KETTLE](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-53-320.jpg)






























![AGENT SPECIFIC FILLING SYSTEMSFILLER RECEPTACLE[FILLER SOCKET/VAPORIZER FILLER UNIT/FILL & DRAIN SYSTEM]MUST PERMIT THE INSERTION OF THE INTENDED BOTTLE.MUST ‘VE A MEANS OF TIGHTENING THE MALE ADAPTER ,TO FORM A TIGHT SEAL.](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-96-320.jpg)


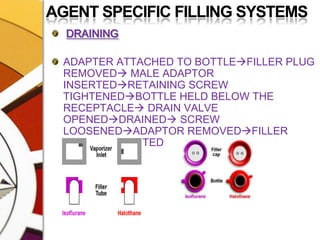
![AGENT SPECIFIC FILLING SYSTEMSFILLINGVAPORIZER TURNED OFF PLUG REMOVEDBOTTLE WITH ADAPTER INSERTED SUCH THAT THE GROOVES MATCH; [TUBE BENT SUCH THAT BOTTLE IS BELOW INLET]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-99-320.jpg)
![AGENT SPECIFIC FILLING SYSTEMSFILLINGRETAINING SCREW TIGHTENEDFILL VALVE [VENT] OPENEDBOTTLE HELD HIGHAIR FROM THE VAPORIZER DISPLACED BY THE LIQUID MOVES THROUGH THE OTHER TUBE AND ENTERS THE AIR SPACE INSIDE THE BOTTLEGENTLE UP AND DOWN MOTION MAY HELP](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-100-320.jpg)









![CLOVER PORTABLE REGULATING ETHER INHALER 1877By Joseph Clover [surgeon ->anesthetist]For ether & chloroformCan regulate the amount of vapor inhaled, rapid onset,no valvesMetal sphere with ether; sphere rotated around a central tube through which patient inhalesModel for Ombredanne’s inhalerMarkings not related to output concentration](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-112-320.jpg)

![VERNON HARCOURT CHLOROFORM INHALER 1903BY AG Vernon HarcourtFor chloroform [amount inhaled <2%,hence safe]Simple, accurate, portableApparatus could be worn around the chloroformist’s neckDanger! Don’t lean to take something from the floor](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-114-320.jpg)
![OMBREDANNE ETHER INHALER 1908Louis Ombredanne [plastic Sxn]Strongly argued for air! Against ether!Criticized Clovers ‘useless’ water chamber Had a modern bag [made from cow’s caecum]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-116-320.jpg)

![SOMNOFORM INHALER 1908SOMNOFORM MIXTURE: Ethyl chloride+ Methyl chloride+ Ethyl bromide [60:35:5]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-118-320.jpg)







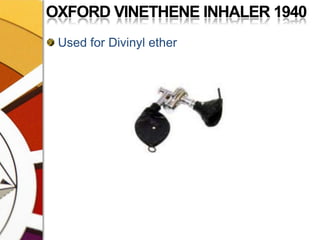
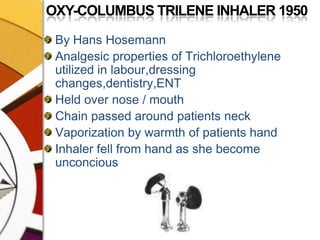




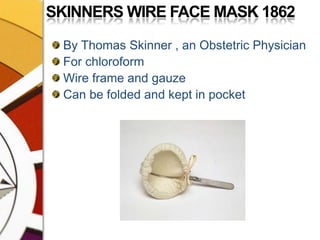








![OXFORD MINIATURE VAPORIZER[OMV]AdvantagesSmall, portable, simple, less servicingMay be drained of one agent & filled with the otherDetachable scales for several agents](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-142-320.jpg)
![OXFORD MINIATURE VAPORIZER[OMV]PARTS:ETHER PLACED IN INNER CONTAINERSURROUNDED BY CRYSTALS OF HYDRATED CaCl₂ MIXING CHAMBERCONTROL VALVE 50 Ml CAPACITYWEIGHT WITH WATER 1060gm](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-143-320.jpg)
![OXFORD MINIATURE VAPORIZER[OMV]hot water jackets causes melting of CaCl₂. It cools and solidifies and relases heat of crystalisation. This heat absorbed by ether vaporisation mixed with O₂ in the mixing chamber deliveredProne to tippingScrew cap fillingBack pressure compensation by circuit valves](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-144-320.jpg)
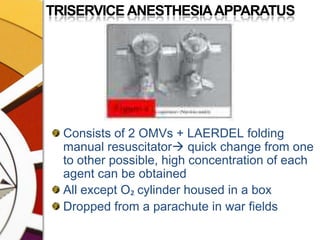
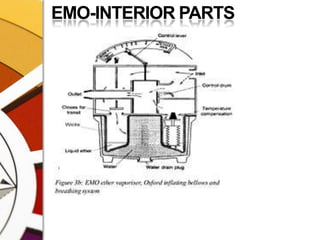
![EPSTEIN MACINTOSH OXFORD VAPORIZER 1952 [EMO]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-146-320.jpg)
![EPSTEIN MACINTOSH OXFORD VAPORIZER 1952 [EMO]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-147-320.jpg)
![EPSTEIN MACINTOSH OXFORD VAPORIZER 1952 [EMO]](https://image.slidesharecdn.com/vaporizers1-100218134044-phpapp01/85/Vaporizers-Basics-148-320.jpg)