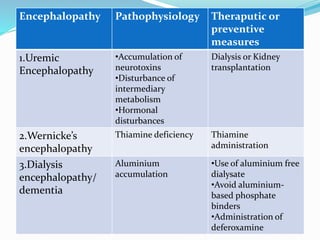
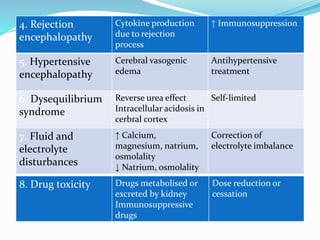
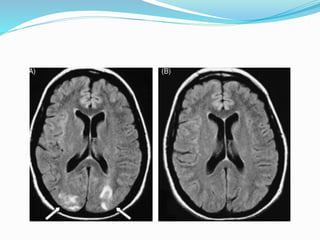
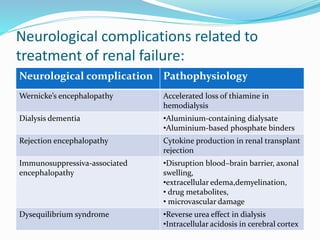
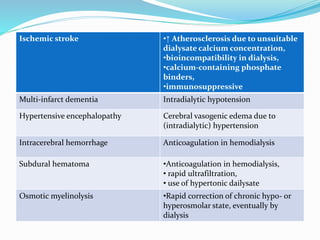
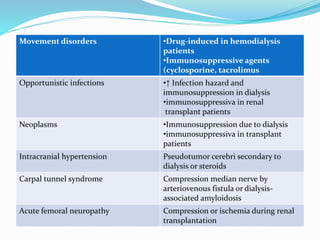
This document summarizes various neurological complications that can occur in patients with renal disease. It discusses central nervous system complications such as encephalopathy, dementia, and cerebrovascular disease. It describes the pathophysiology and treatment of various types of encephalopathy including uremic encephalopathy, Wernicke's encephalopathy, and dialysis encephalopathy. It also discusses peripheral nervous system complications including mononeuropathy, polyneuropathy, and myopathy.