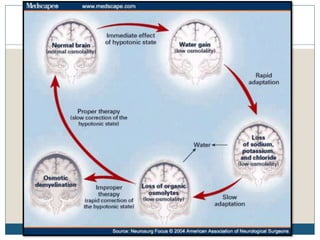
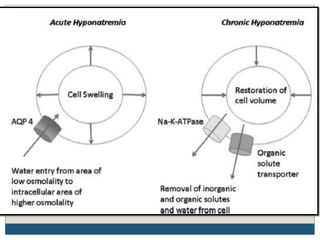
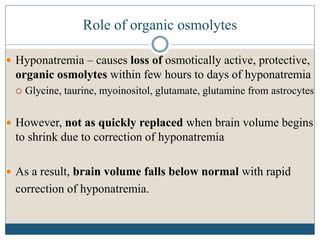
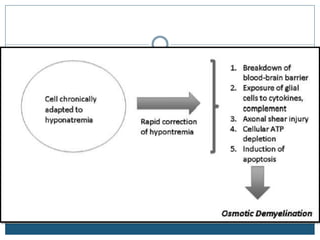
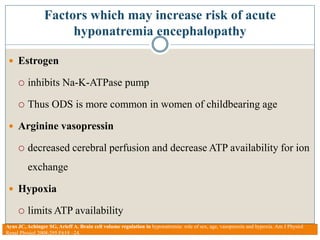
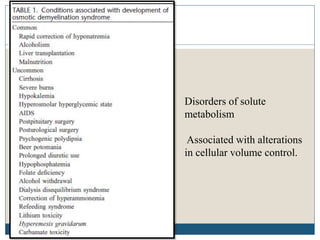
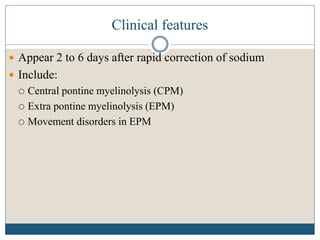
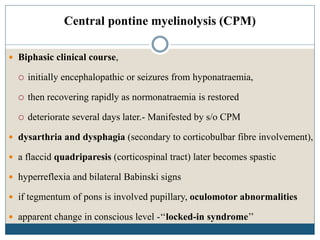
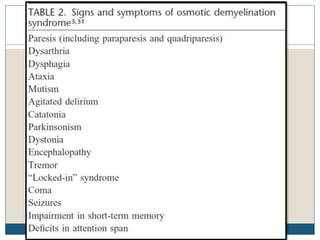
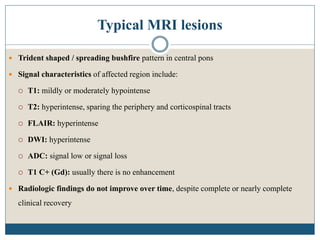
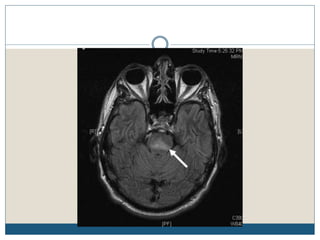
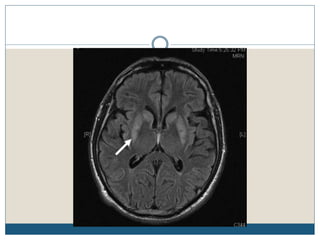
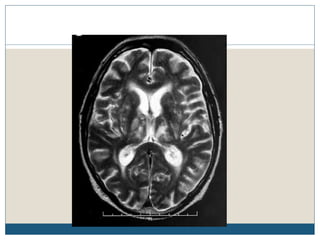
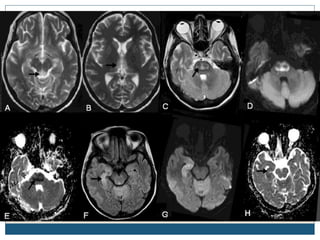
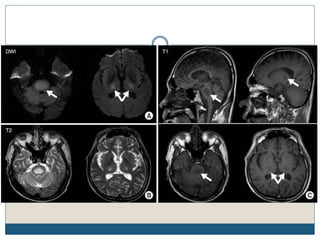
This document provides an overview of osmotic demyelination syndrome (ODS), also known as central pontine myelinolysis. It discusses the history, controversies in nomenclature, pathology, epidemiology, pathophysiology, clinical features, diagnosis, management including prevention, re-lowering sodium levels, supportive care and investigational therapies, prognosis, and key references. The document is intended as an educational resource for physicians on ODS.