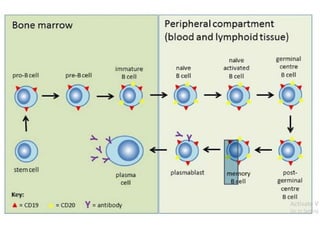
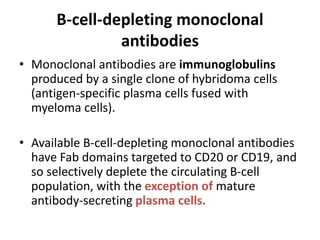
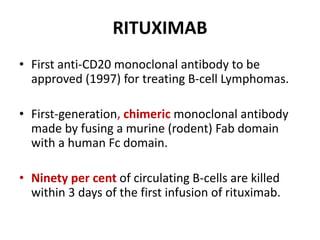
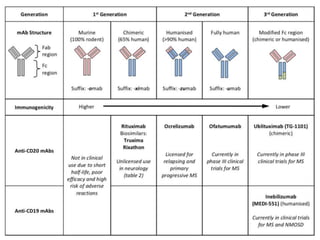
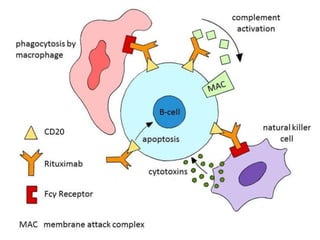
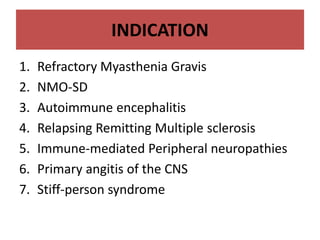
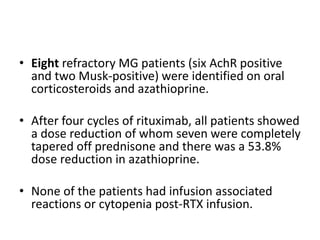
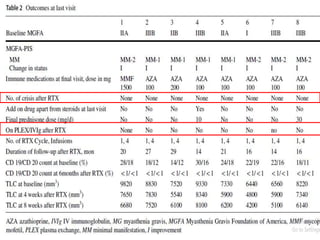
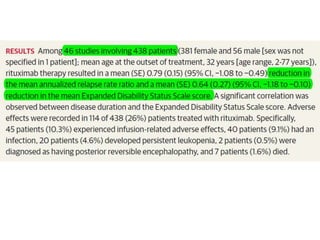
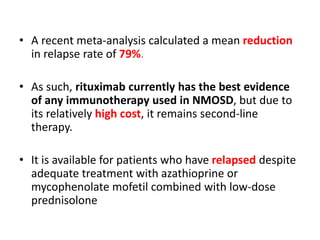
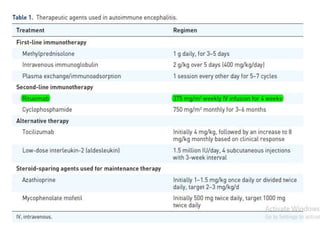
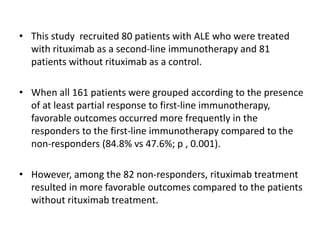
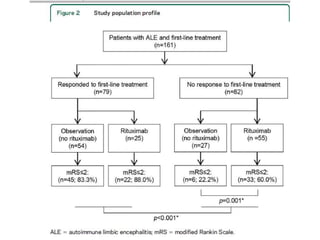
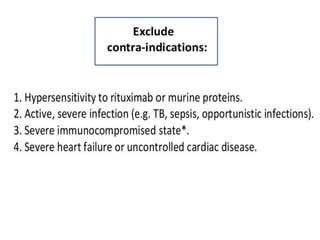
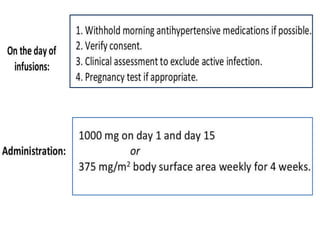
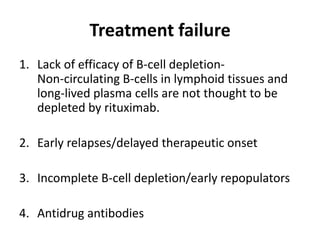
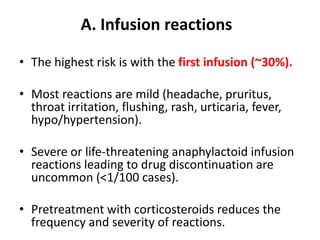
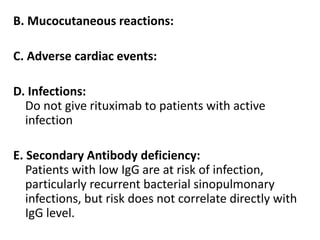
Rituximab is a monoclonal antibody that targets B cells. It is used to treat various neurological conditions associated with autoantibodies or B cell dysfunction, including refractory myasthenia gravis, NMOSD, autoimmune encephalitis, and relapsing MS. The document discusses B cell biology, mechanisms of action of rituximab, indications, dosing, efficacy evidence, risks and monitoring considerations for rituximab's use in neurology. Key risks include infusion reactions and secondary infections due to prolonged B cell depletion.