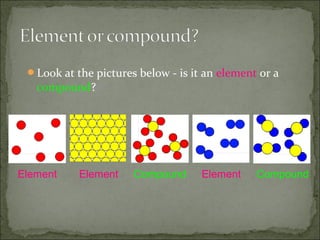
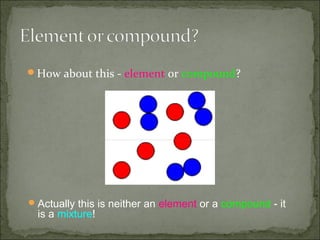
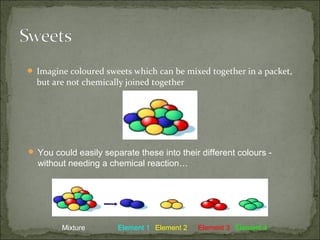
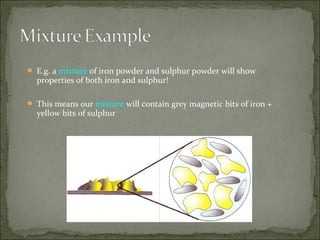
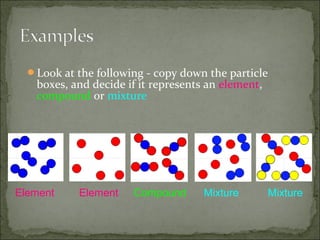
This document defines and distinguishes between elements, compounds, and mixtures. It notes that an element contains only one type of atom, a compound contains two or more types of atoms chemically bound together, and a mixture contains two or more substances that are not chemically bound and can be easily separated. Examples of each are provided, including air as a mixture of gases and a mixture of iron and sulfur powders showing properties of both.