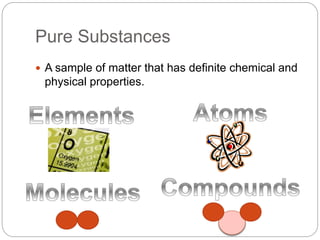
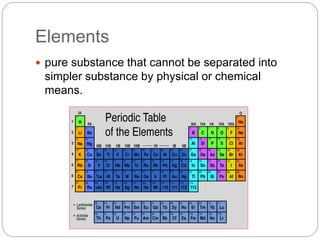
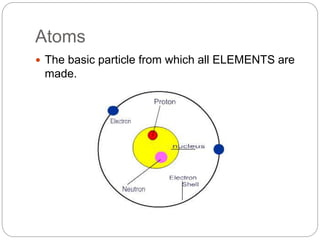
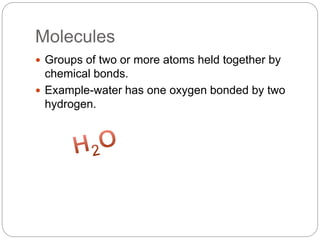
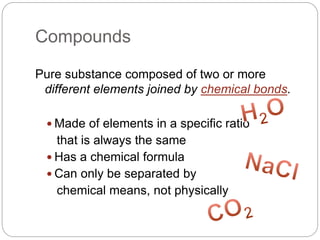
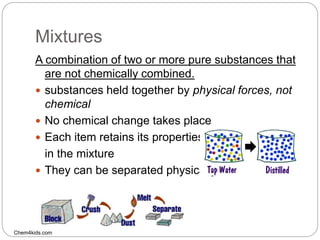
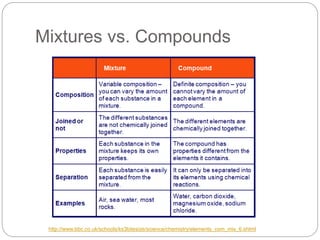
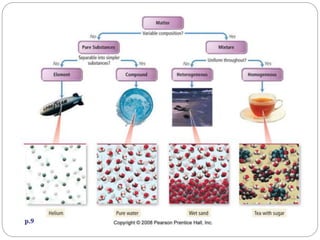
This document discusses the classification of matter into elements, compounds, and mixtures. It defines an element as a pure substance made of only one type of atom that cannot be separated into simpler substances. Compounds are pure substances composed of two or more elements chemically bonded together in specific ratios. Mixtures are physical combinations of substances that are not chemically bonded and can be separated by physical means. The document provides examples of elements, compounds and mixtures, and uses a series of pictures to have the reader identify whether each pictured substance is an element, compound or mixture.