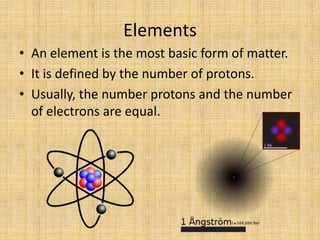
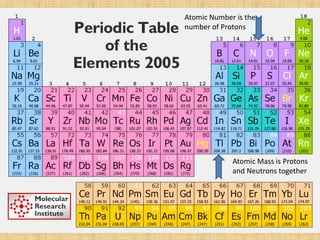
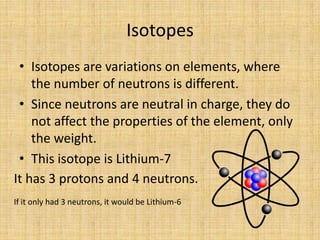
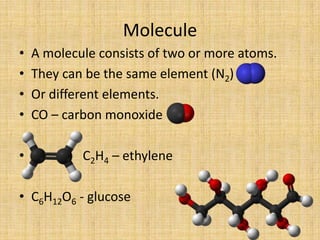
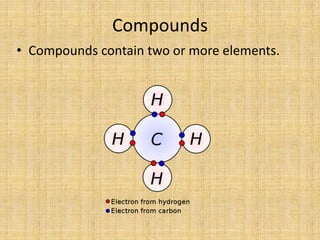
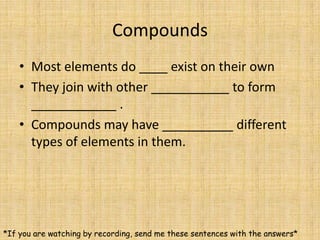
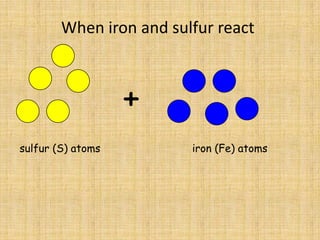
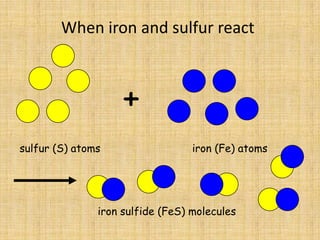
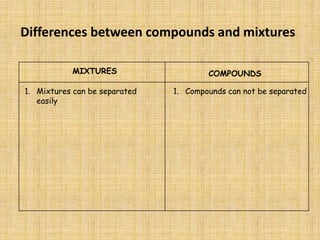
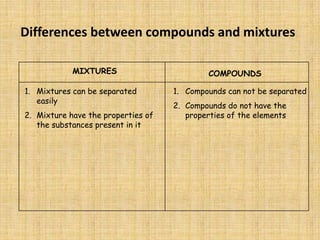
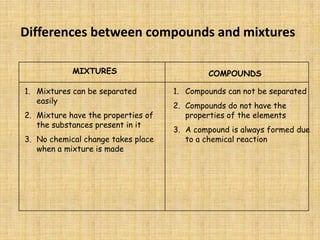
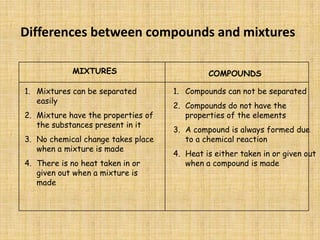
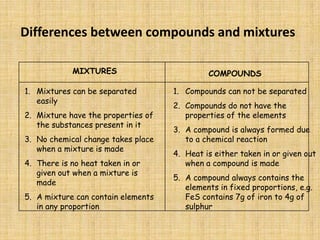
Jeff Taylor teaches physical science courses. He lives in a 22 foot trailer with his wife Linda and 19-month old daughter Hilina. They are spending the next 6 months traveling around the Desert Southwest, detailing their travels on two blogs. The document then provides information about the structure of matter, including protons, neutrons, electrons, elements, isotopes, molecules, compounds, mixtures, solutions and chemical reactions.