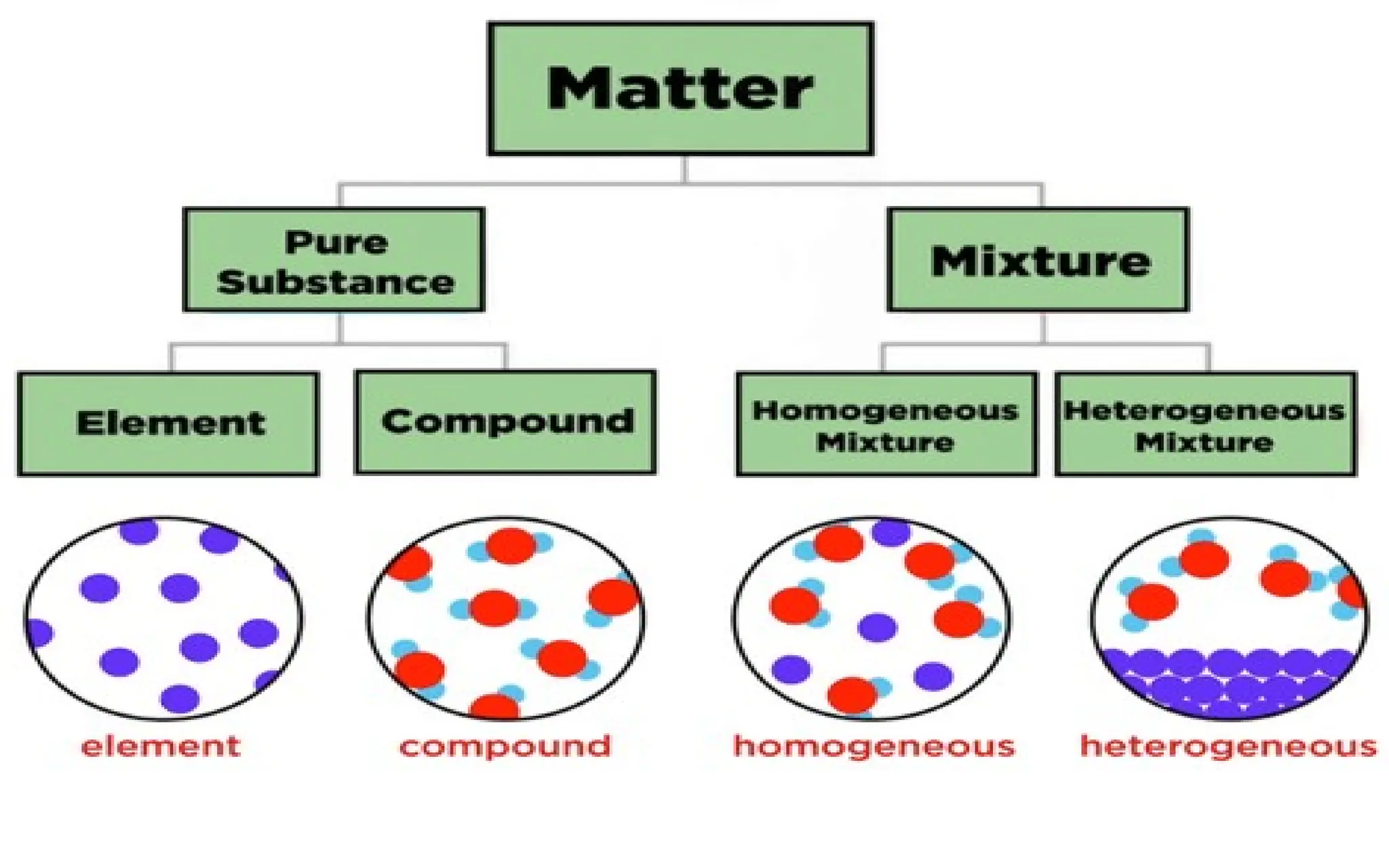
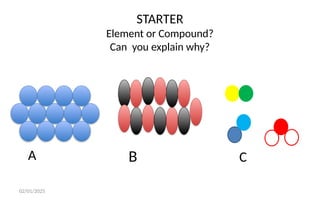
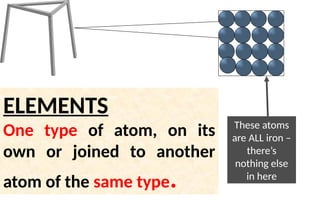
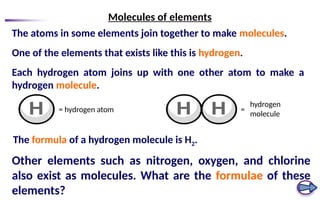
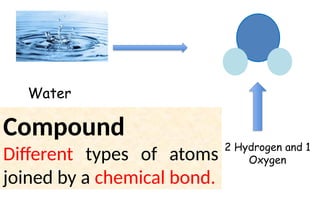
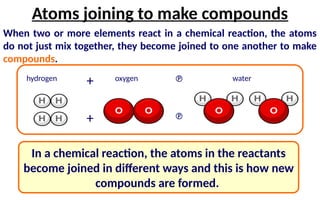
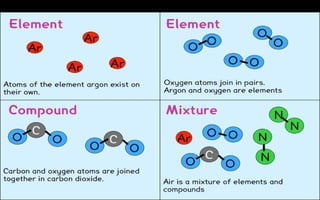
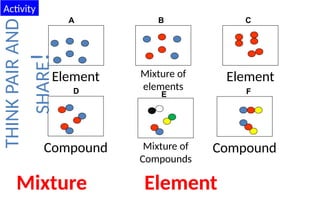
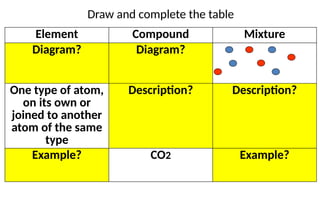
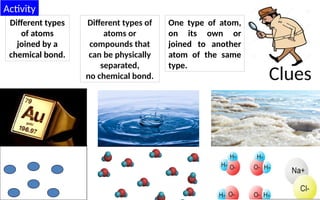
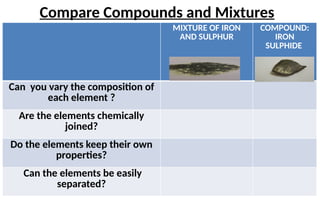
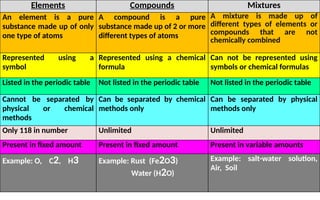
The document defines elements, compounds, and mixtures, highlighting their characteristics and differences. Elements consist of a single type of atom, compounds are formed by two or more types of atoms bonded together, and mixtures comprise different substances that can be physically separated. It also includes examples and comparisons to help understand the concepts better.