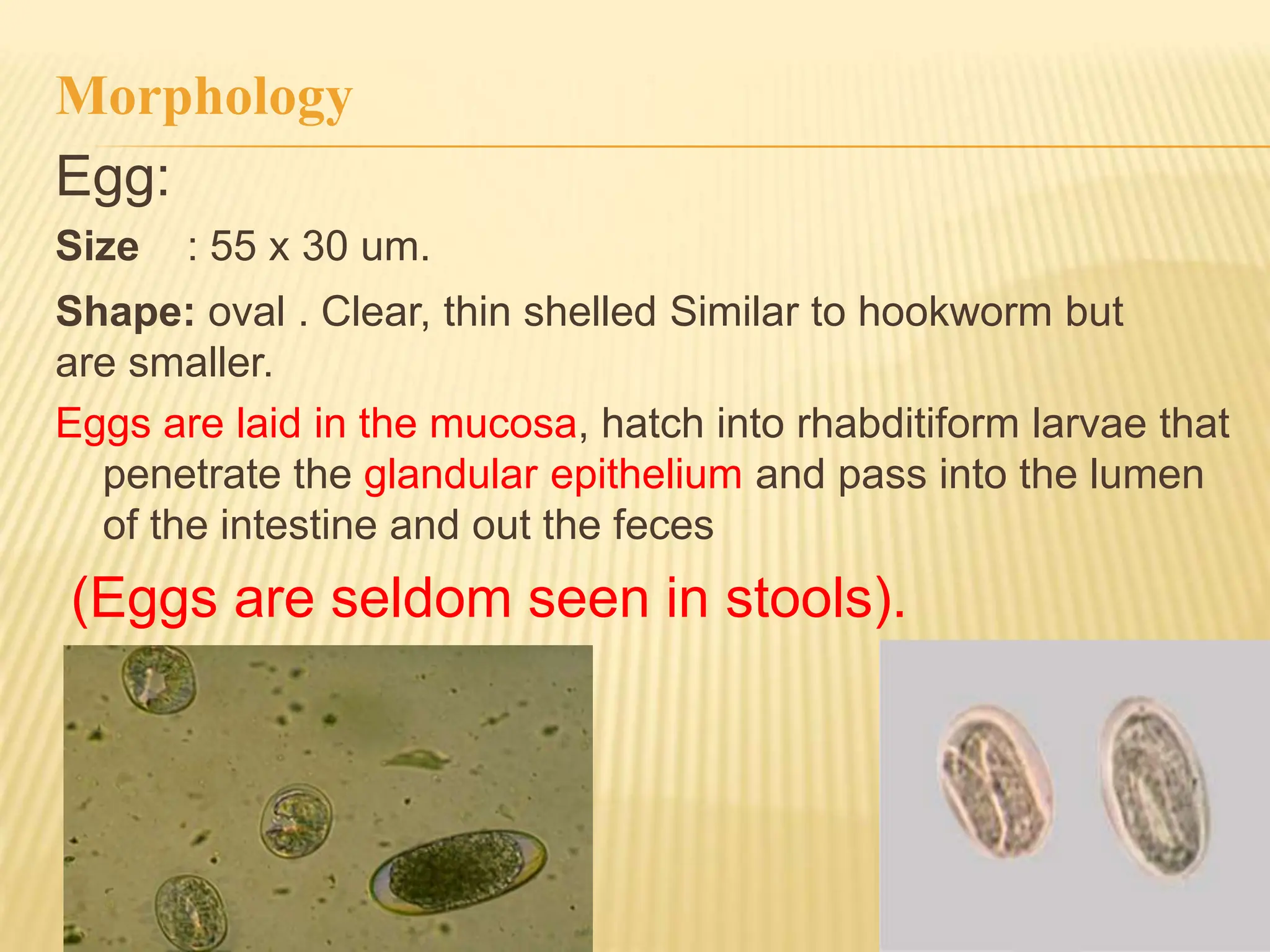
Strongyloides stercoralis is a soil-transmitted helminth parasite with both free-living and parasitic life cycles. In its parasitic cycle, the filariform larvae penetrate the skin and travel to the lungs before migrating up the respiratory tract and into the small intestine where females reside. The females lay eggs which hatch into rhabditiform larvae that pass in the feces but can also develop into filariform larvae, allowing reinfection of the same host. Heavy infections can suppress the immune system and cause disseminated strongyloidiasis, a potentially fatal condition. Diagnosis involves finding larvae in stool or biopsy samples.