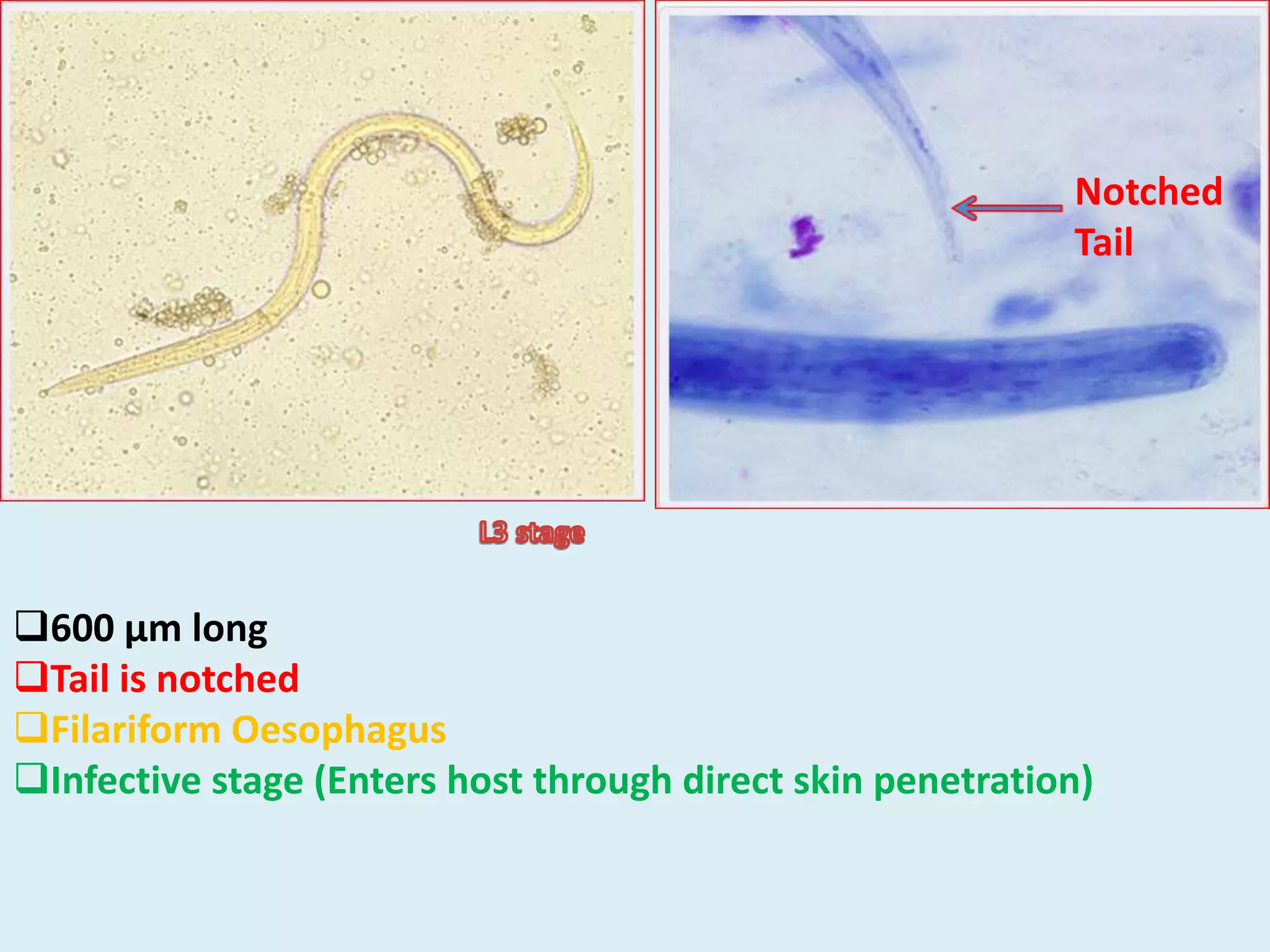
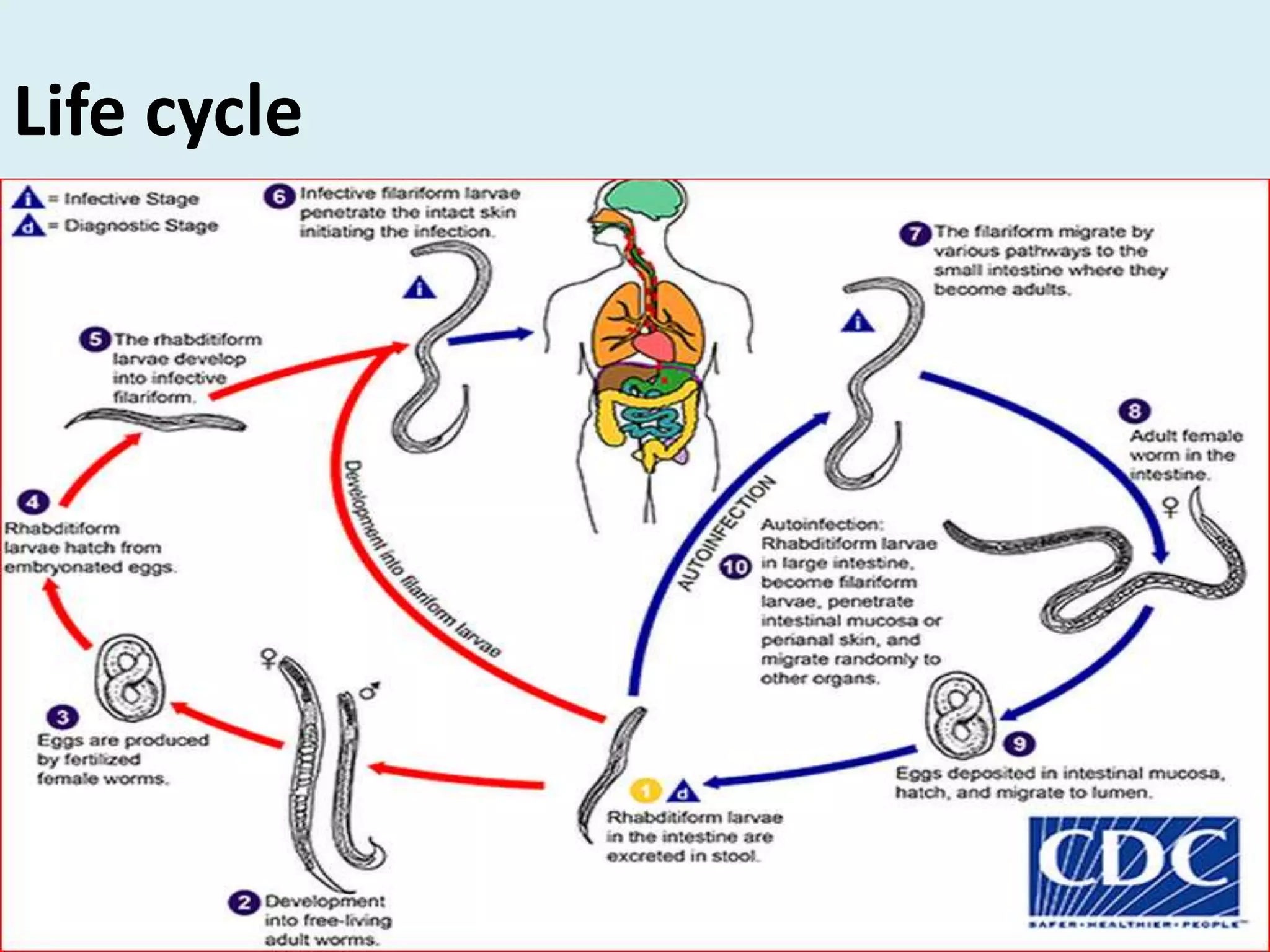
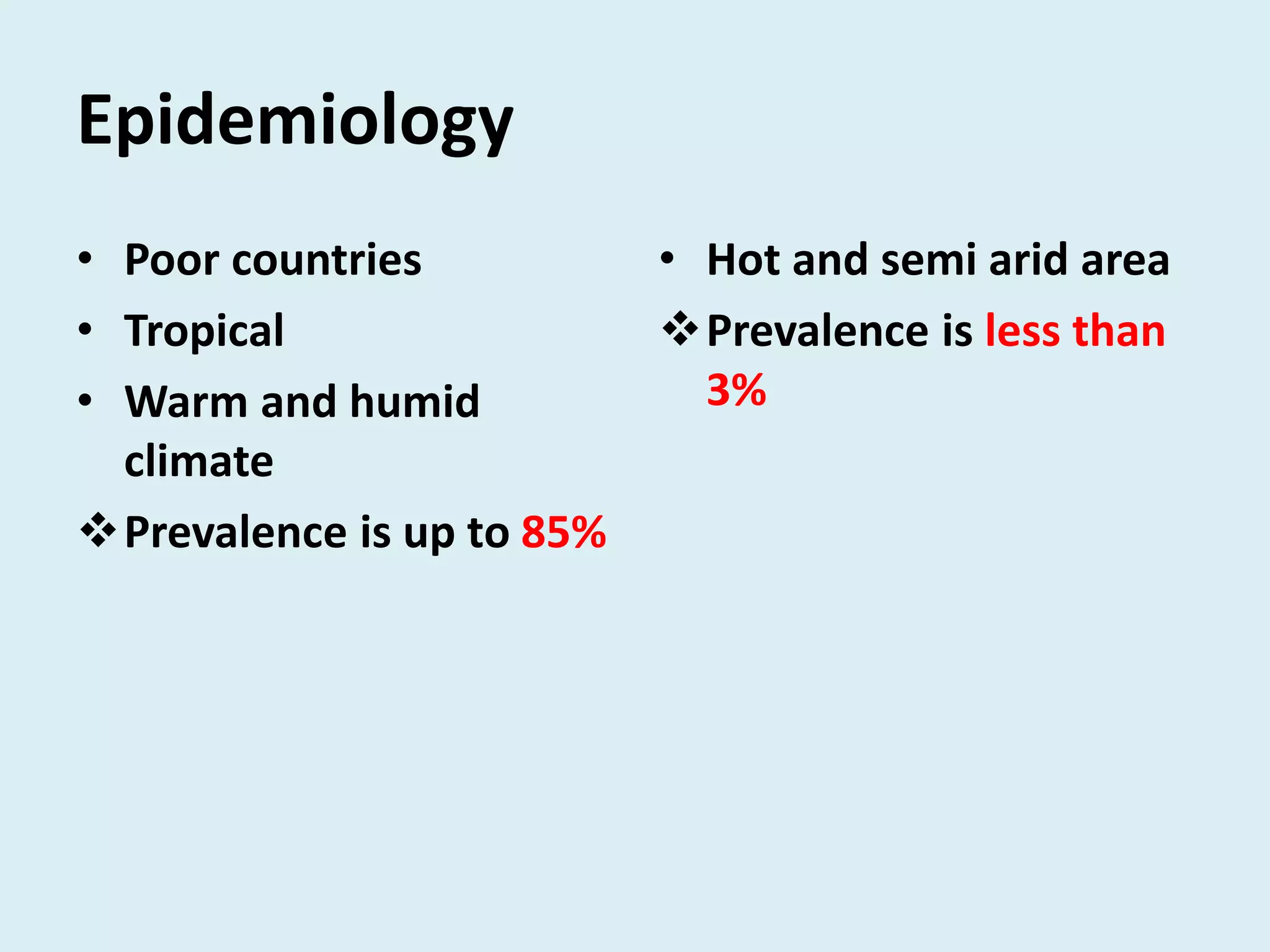
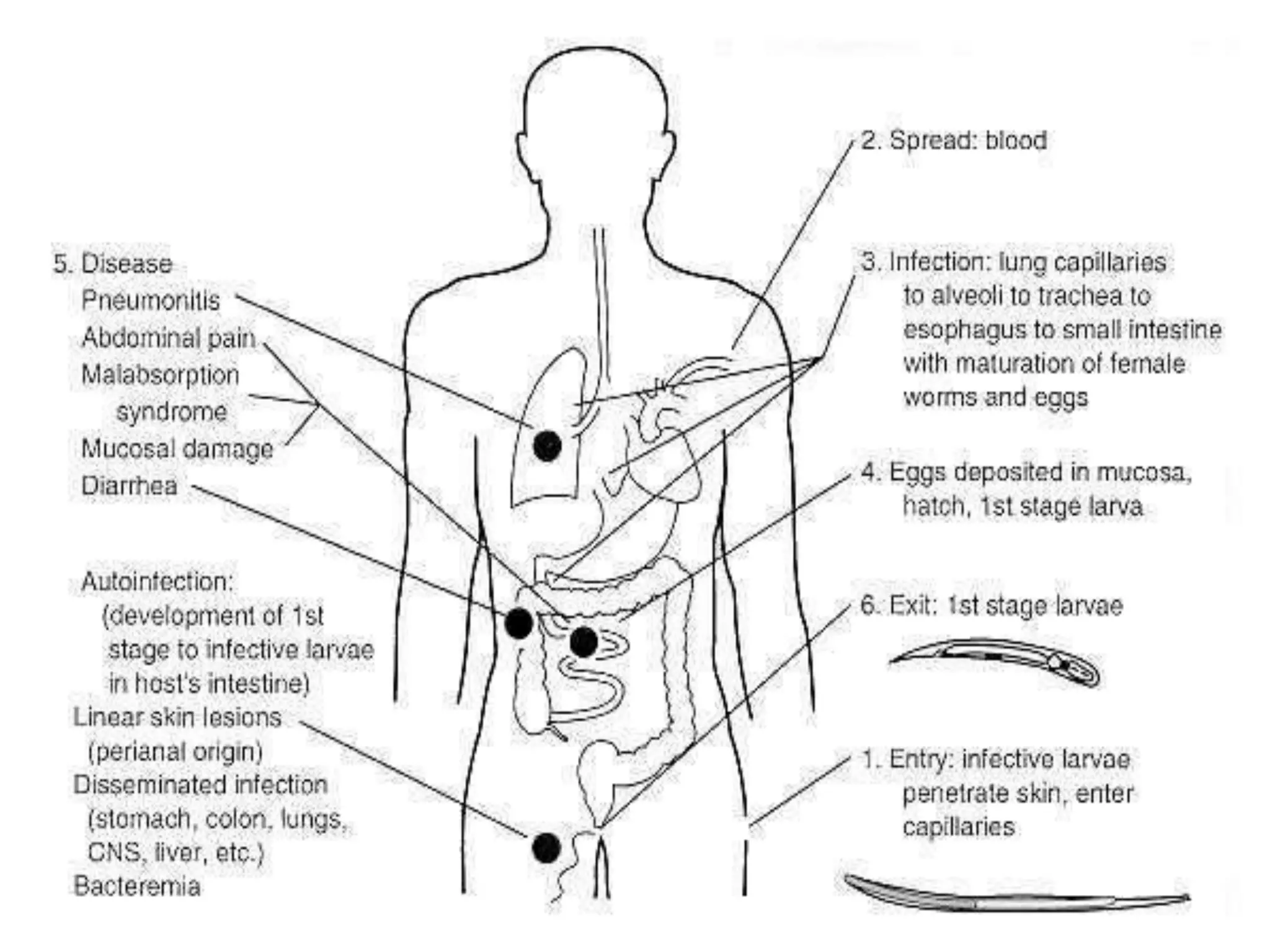
Strongyloides is a genus of parasitic roundworms that can infect humans and other primates. It causes strongyloidiasis in humans through skin penetration of the infective filariform larval stage. The infection may remain asymptomatic but can become life-threatening if it spreads throughout the body (disseminated) in immunocompromised individuals. Diagnosis involves detecting larvae in stool samples. Treatment involves anthelmintic drugs like ivermectin. Prevention relies on good hygiene practices to avoid skin contact with contaminated soil.